Research
PREVIOUS PROJECTS
In vitro differentiation of endothelial progenitor cells from human umbilical cord blood as a support for neovascularization.
Bone marrow-derived stem cell therapy for vascular regeneration and reconstruction.
Development of cell therapies using en dothelial progenitors derived from human fetal tissues.
The expression of HDAC inhibitors on endothelial progenitor cells.
Effects of plant lectin on endothelial pro genitors.
Set up a stem cells bank for research and autologous transplant.
CURRENT PROJECTS
Epigenetic mechanisms involved in the differentiation and migration of endothelial progenitor cells: new tools for vascular cell therapy (Fig.1, 2 and 4)
Fetal umbilical cord derived mesenchymal stem cells (MSC) can differentiate into various cell types including endothelial cells, which may be valuable in cellular therapy of cardiovascular diseases. However, their ability for cardiovascular tissue repair has not been elucidated.
Aims: (i) evaluation of the angiogenic and regenerative potential of human fetal endothelial progenitor cells (EPC) employing the experimental model of human vascular tissue slices (huVTS); (ii) investigation of the role of Hox transcription factor modulation.
The final goal is to understand the micro-environmental interactions that regulate MSC differentiation, and to improve the efficiency of EPC homing to the injured sites.
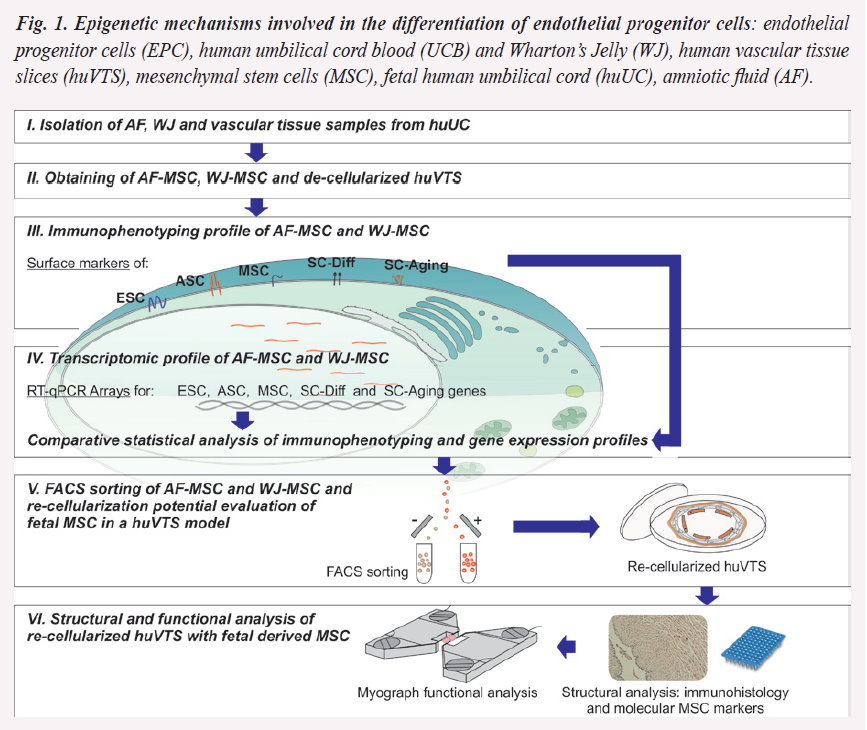
Objectives: (1) Using selected populations of MSC collected from human umbilical cord blood (UCB) and Wharton’s Jelly (WJ), we will evaluate their differentiation status assessing the methylation of endothelial genes and acetylation of Hox transcription factors. (2) Integration capacity of UCB- and WJ-derived EPC on human fetal? vessels.
To test the involvement of histone deacetylases (HDAC) activity in endothelial lineage progression we investigated the effects of HDAC inhibitors on UCB-derived EPCs. Adherent EPCs that expressed the endothelial marker proteins (PCAM, CD105, CD133, and VEGFR2) were treated with 3 HDAC inhibitors: Butyrate (BuA), Trichostatin A (TSA), and Valproic acid (VA).
Preliminary results: RT-PCR assay showed that HDAC inhibitors down-regulated the expression of endothelial genes (VE-cadherin, CD133, CXCR4 and Tie-2) and selectively reduce the expression of VEGFR2, CD117, VE-cadherin, and ICAM-1, whereas the expression of CD45 and CD34 remained unchanged.
Real-Time PCR showed that TSA down-regulated telomerase activity probably via suppression of hTERT expression, suggesting that HDAC inhibitor decreased cell proliferation and cell motility (wound-healing assay). The balance of acethylation/deacethylation kept in control by the activity of HAT (histone acetyltransferases)/HDAC enzymes plays an important role in differentiation of SC by regulating proliferation and endothelial lineage commitment.
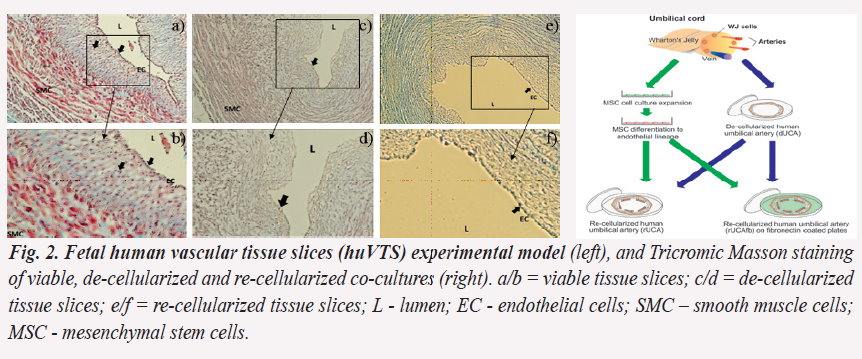
A preliminary evaluation of huVTS indicate a re-cellularization process (c/f) comparatively with viable and de-cellularized samples (a/d):
Perspectives to demonstrate the therapeutic potential of MSC, we will identify and test the efficiency of re-cellularized EPC to the injured sites of huVTS. We also plan to confirm that HDAC is involved in endothelial differentiation of progenitor cells.
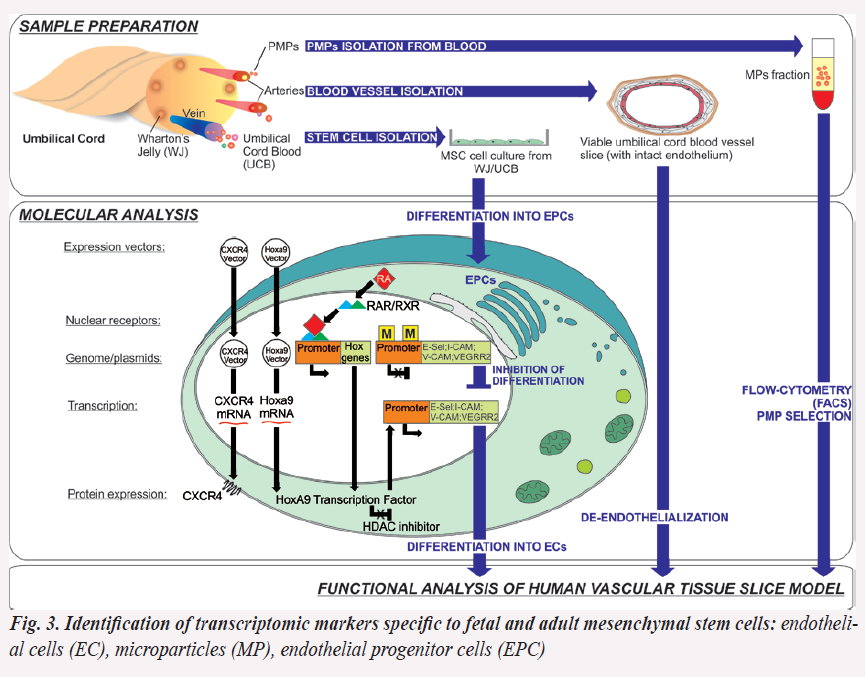
Identification of novel transcriptomic markers specific to fetal and adult mesenchymal stem cells (SC) with therapeutic potential in tissue engineering (Fig. 3 and 4)
Despite the recognized medical value of fetal SC, the archetype of which remains the MSC, the understanding of their native identity, tissue distribution, development frequency and senescence program is not completely known.
Aims: (i) to isolate and cultivate adherent population of human MSC of human fetal origin: umbilical cord (UC) and vessels and amniotic fluid (AF), (ii) to establish the immunophenotypic and molecular stemness profile in relation with their specific resident markers, their differentiation potential and senescence profile.
Objectives: (1) to identify, culture and sort to homogeneity by flow cytometry of amniotic fluid stem cells (AFSC) and UC-derived MSC; (2) to establish the immunophenotypic and transcriptomic profile, and (3) set up the structural and functional analysis of AF derived MSC, (4)- to compare the immunophenotyping with molecular marker analysis of fetal MSC compared with cryopreserved adult bone marrow (BM) samples.
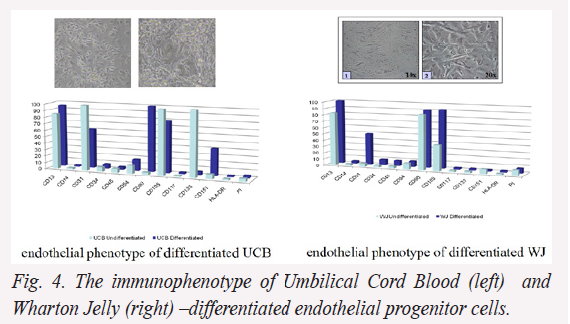
Preliminary data: during differentiation the cells isolated from UCB demonstrate a decrease of hematopoietic surface markers expression, as well as an increase of endothelial specific markers (CD31, CD105, CD133, CD151), and stromal SC markers (CD13 and CD90). In comparison, cells isolated from WJ exhibit a better endothelial differentiation capacity sustained by the increased expression of specific antigens such as CD13, CD31, and CD105.
Perspectives: We will investigate the transcriptomic profile of AFSC. Besides the therapeutic potential of the MSC, we plan to test the theory/hypothesis of the dual marker phenotype of fetal SC, embryonic and adult, and based on the PCR array we will obtain data on the transcriptomic profile and the origins of fetal MSC.
Personalized treatment with mesenchymal stem cells for steroid-refractory Graft versus Host Disease after allogeneic hematopoietic stem cell transplantation (Fig. 5)
A major therapeutic strategy for hematological malignancies is hematopoietic stem cells (HSC) transplantation. However, graft-versus-host disease (GvHD) and transplant rejection are life-threatening situations in SC transplant. The project’s concept is that personalized therapy with in vitro expanded and characterized bone marrow derived MSC (BM-MSC) could decrease the severe reaction and immunological response in steroid treatment-resistant GvHD after SC transplantation.
Aim: to improve BM-MSC treatments and implement MSC therapy for HD patients with steroid refractory GvHD, after allogeneic HSC transplantation as a personalized treatment meant to eliminate the severe reaction and increase donor HSC migration and reconstitution potential into the recipients.
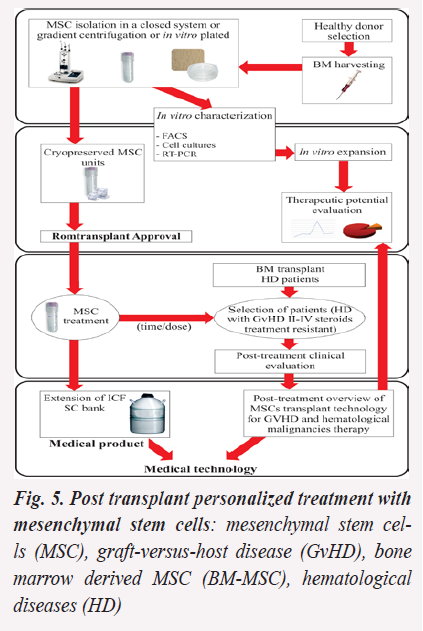
Objectives: (1) to establish a paraclinical data based algorithm in order to evaluate MSC therapeutic potential; (2) to elaborate a Romanian MSC transplant data register, in accordance with the European Group for Blood and Bone Marrow Transplantation guidelines. (3) to obtain the accreditation for MSC units as medical product and for MSC treatment as medical service.
Preliminary data: The comparative immunophenotyping of human BM-MSC units revealed similar profiles at revitalizing in comparison with the harvesting moment: CD1alow/CD10+/CD11b-/CD13+/CD14-/CD29+/CD31-/CD34-/CD38-/CD44+/CD45-/CD49a/blow/ CD54-/CD56low/CD73+/CD90+/CD105+/CD117-/CD133-/CD144-/CD146+/ and HLA-ABC+/-DR- profiles.
Perspectives: We will elaborate standard operation protocols (SOP) for harvesting, isolation, testing and characterizing human SC units and healthy BM donor’s selection. The results of our studies will provide prediction scores for the evaluation of human BM-MSC therapeutic potential.
After obtaining the agreement of MSC treatment for HD patients with severe steroid-refractory GvHD, we will set a MSC treatment guide for hematological patients developing GvHD after BM allotransplant. This will increase the probability of a positive clinical outcome (symptomatology remission) and will be the frame for the validation of MSC treatment as medical service and MSC unit as medical product.
We will continue the organization of the stem cell bank as a source of material for basic research and clinical use in cellular transplant.

