DEP
Inflammation Laboratory |
|||||||
|
|
|||||||
Research Interest: |
Our research is centered on understanding the mechanisms of cell-to-cell communication and the regulation of inflammation at various post-transcriptional levels of gene expression. We explore these processes across a range of cardiovascular in vivo and in vitro models, including atherosclerosis, myocardial infarction, diabetes, cardiac fibrosis, and valvular pathology. |
||||||
Main Research Topics: |
1. Role of immune cells in cardiac fibrosis.
|
||||||
Our Team: |
 |
||||||
Contact us: |
Elena Butoi, Head of Inflammation Department (CV-link)elena.dragomir@icbp.roMonica Ţucureanu, Lab. PI (CV-link)monica.pirvulescu@icbp.ro |
||||||
Previous Grants: |
1. 2022-2024: PN-III-P1-1.1-PD-2021-0498 – “Impact of high glucose in valvular endothelial cells-monocyte crosstalk: molecular signatures and role in early valvular dysfunction” - VALDYSIGN2. 2020-2023: ERA-NET NEURON Call for Joint Transnational Research Projects – “Stroke risk prediction in atherosclerosis measuring circulating complement system proteins” - STATEMENT3. 2020-2022: PN-III-P2-2.1-PED-2019-4906 – “Development and validation of a native cardiac hydrogel for myocardial repair post-infarction” – NAMIGEL4. 2018-2022: PN-III-P4-ID-PCCF-2016-0172 – “Targeting innate immune mechanisms to improve risk stratification and to identify future therapeutic options in myocardial infarction” – INNATE-MI5. 2018-2021: PCCDI Complex Project nr. 13 PCCDI/2018 "Intelligent therapies for non-communicable diseases based on controlled release of pharmacological compounds from encapsulated engineered cells and targeted bio nanoparticles" – INTERA6. 2016-2020: Competitiveness Operational Programme, Priority Axis 1/Action 1.1.4 “Targeted therapies for diabetes-related aortic valve disease” – THERAVALDIS7. 2015-2017: PN-II-RU-TE-2014-4-0965 – Vascular cell cross-talk, induces specific microRNAs that can be relevant for atherosclerotic plaque rupture, in type 2 diabetes patients – MIRDIATRIX8. 2011-2016: PN-II-ID-PCE-2011-3 – Molecules and mechanisms involved in cytokine and chemokine-dependent vascular inflammation as targets for novel nano-therapeutic strategies, Exploratory Research Projects – REFINATER9. 2011-2014: European Innovative RTD Projects Proposals in Nanomedicine EuroNanoMed JTC 2010, FP7,“Nanoparticles designed to target chemokine-related inflammatory processes in vascular diseases and cancer metastasis and implementation of a biosensor to diagnose these disorders” – NANODIATER |
||||||
Recent Results |
|||||||
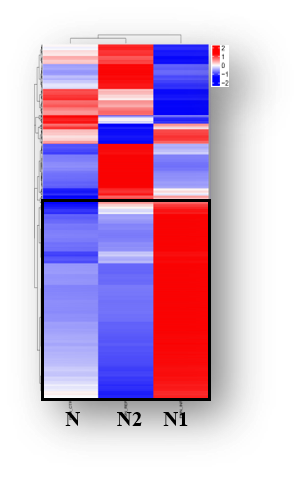
Mihaila et al.; Front. Immunol., 12:708770., 2021
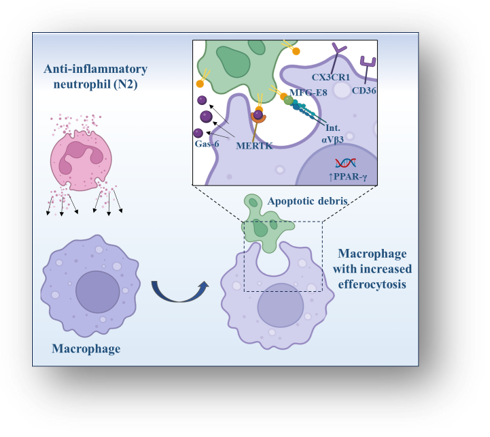
Mihaila et al.; Cells. Jan 23;13(3):208., 2024
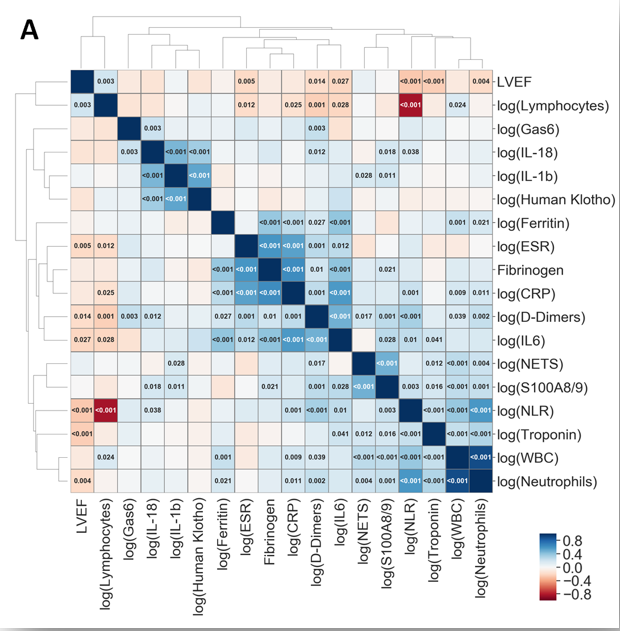
Barbu et al.; Int J Mol Sci.;25(10):5107, 2024
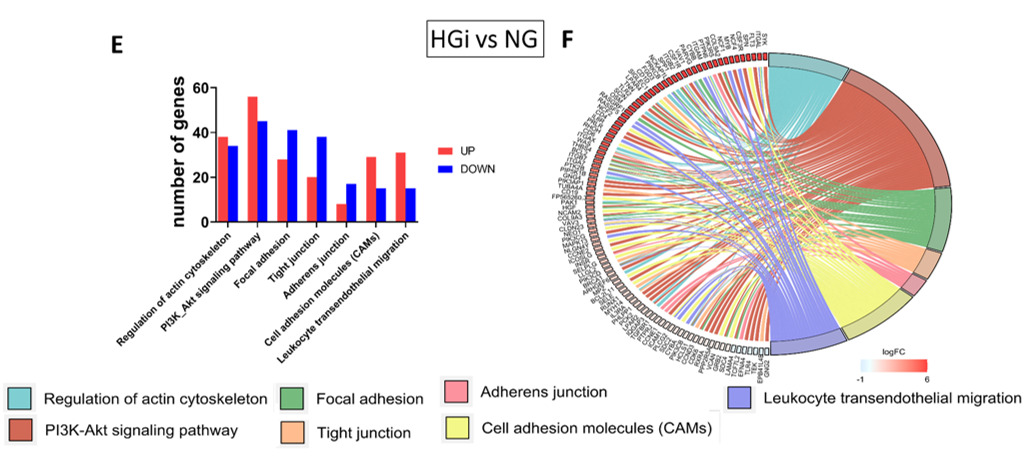
Tucureanu et al.; Int J Mol Sci.; 25(5):3048, 2024
|
|||||||
Publications:(last 5 years) |
1. Barbu E, Mihaila AC, Gan AM, Ciortan L, Macarie RD, Tucureanu MM, Filippi A, Stoenescu AI, Petrea SV, Simionescu M, Balanescu SM, Butoi E. The Elevated Inflammatory Status of Neutrophils Is Related to In-Hospital Complications in Patients with Acute Coronary Syndrome and Has Important Prognosis Value for Diabetic Patients. Int J Mol Sci. 2024 May 8;25(10):5107. IF: 5.6 2. Mihaila AC, Ciortan L, Tucureanu MM, Simionescu M, Butoi E. Anti-Inflammatory Neutrophils Reprogram Macrophages toward a Pro-Healing Phenotype with Increased Efferocytosis Capacity. Cells. 2024 Jan 23;13(3):208. IF: 6 3. Tucureanu MM, Ciortan L, Macarie RD, Mihaila AC, Droc I, Butoi E, Manduteanu I. The Specific Molecular Changes Induced by Diabetic Conditions in Valvular Endothelial Cells and upon Their Interactions with Monocytes Contribute to Endothelial Dysfunction. Int J Mol Sci. 2024 Mar 6;25(5):3048. IF: 5.6 4. Macarie RD, Tucureanu MM, Ciortan L, Gan AM, Butoi E*, Mânduțeanu I*. Ficolin-2 amplifies inflammation in macrophage-smooth muscle cell cross-talk and increases monocyte transmigration by mechanisms involving IL-1β and IL-6. Sci Rep. 2023 Nov 8;13(1):19431. IF: 4.6 5. Nicu R, Ciolacu DE, Petrovici AR, Rusu D, Avadanei M, Mihaila AC, Butoi E, Ciolacu F. 3D Matrices for Enhanced Encapsulation and Controlled Release of Anti-Inflammatory Bioactive Compounds in Wound Healing. Int J Mol Sci. 2023; 20;24(4):4213. IF: 6.208 6. Vadana M, Cecoltan S, Ciortan L, Macarie RD, Mihaila AC, Tucureanu MM, Gan AM, Simionescu M, Manduteanu I, Droc I, Butoi E. Parathyroid Hormone Induces Human Valvular Endothelial Cells Dysfunction That Impacts the Osteogenic Phenotype of Valvular Interstitial Cells. Int J Mol Sci. 2022 Mar 29;23(7):3776. IF: 6.208 7. Sanz CG, Mihaila AC, Evanghelidis A, Diculescu VC, Butoi E, Barsan MM. Quantification of cell oxygenation in 2D constructs of metallized electrospun polycaprolactone fibers encapsulating human valvular interstitial cells. Journal of Electroanalytical Chemistry. Volume 905, 15 January 2022, 116005, IF: 4.46 8. Mihaila AC, Ciortan L, Macarie RD, Vadana M, Cecoltan S, Preda MB, Hudita A, Gan AM, Tucureanu MM, Simionescu M, Schiopu A and Butoi E. Transcriptional profiling and functional analysis of N1/N2 neutrophils reveal an immunomodulatory effect of S100A9-blockade on the pro-inflammatory N1. Front. Immunol., 2021, doi: 10.3389/fimmu.2021.708770. IF: 8.78 9. Cecoltan S, Ciortan L, Macarie RD, Vadana M, Mihaila AC, Tucureanu MM, Vlad ML, Droc I, Gherghiceanu M, Simionescu A, Simionescu DT, Butoi E and Manduteanu I. High glucose induced changes in human VEC phenotype in a 3D hydrogel derived from cell-free native aortic root. Front. Cardiovasc. Med., 2021 Aug 12;8:714573. IF: 6.05 10. Ciortan L, Macarie RD, Cecoltan S, Vadana M, Tucureanu MM, Mihaila AC, Droc I, Butoi E, Manduteanu I. Chronic High Glucose Concentration Induces Inflammatory and Remodeling Changes in Valvular Endothelial Cells and Valvular Interstitial Cells in a Gelatin Methacrylate 3D Model of the Human Aortic Valve. Polymers (Basel). 2020 Nov 25;12(12):2786. doi: 10.3390/polym12122786. IF: 4.32 11. Wu X, Niculite CM, Preda MB, Rossi A, Tebaldi T, Butoi E, White MK, Tudoran OM, Petrusca DN, Jannasch AS, Bone WP, Zong X, Fang F, Burlacu A, Paulsen MT, Hancock BA, Sandusky GE, Mitra S, Fishel ML, Buechlein A, Ivan C, Oikonomopoulos S, Gorospe M, Mosley A, Radovich M, Davé UP, Ragoussis J, Nephew KP, Mari B, McIntyre A, Konig H, Ljungman M, Cousminer DL, Macchi P, Ivan M. Regulation of cellular sterol homeostasis by the oxygen responsive noncoding RNA lincNORS. Nat Commun. 2020 Sep 21;11(1):4755. IF: 14.92 12. Vadana M, Cecoltan S, Ciortan L, Macarie RD, Tucureanu MM, Mihaila AC, Droc I, Butoi E, Manduteanu I. Molecular mechanisms involved in high glucose-induced valve calcification in a 3D valve model with human valvular cells. J Cell Mol Med. 2020 Jun;24(11):6350-6361. IF: 5.31 13. Tucureanu MM, Filippi A, Alexandru N, Ana Constantinescu C, Ciortan L, Macarie R, Vadana M, Voicu G, Frunza S, Nistor D, Simionescu A, Simionescu DT, Georgescu A, Manduteanu I. Diabetes-induced early molecular and functional changes in aortic heart valves in a murine model of atherosclerosis. Diab Vasc Dis Res. 2019 Nov;16(6):562-576. IF: 4.2 |
||||||
Current fundings: |
|
||||||
Previous Grants: |
1. 2022-2024: PN-III-P1-1.1-PD-2021-0498 – “Impact of high glucose in valvular endothelial cells-monocyte crosstalk: molecular signatures and role in early valvular dysfunction” - VALDYSIGN 2. 2020-2023: ERA-NET NEURON Call for Joint Transnational Research Projects – “Stroke risk prediction in atherosclerosis measuring circulating complement system proteins” - STATEMENT 3. 2020-2022: PN-III-P2-2.1-PED-2019-4906 - “Development and validation of a native cardiac hydrogel for myocardial repair post-infarction” – NAMIGEL 4. 2018-2022: PN-III-P4-ID-PCCF-2016-0172 - “Targeting innate immune mechanisms to improve risk stratification and to identify future therapeutic options in myocardial infarction” – INNATE-MI 5. 2018-2021: PCCDI Complex Project nr. 13 PCCDI/2018 "Intelligent therapies for non-communicable diseases based on controlled release of pharmacological compounds from encapsulated engineered cells and targeted bio nanoparticles" – INTERA 6. 2016-2020: Competitiveness Operational Programme, Priority Axis 1/Action 1.1.4 “Targeted therapies for diabetes-related aortic valve disease” – THERAVALDIS 7. 2015-2017: PN-II-RU-TE-2014-4-0965 - Vascular cell cross-talk, induces specific microRNAs that can be relevant for atherosclerotic plaque rupture, in type 2 diabetes patients – MIRDIATRIX 8. 2011-2016: PN-II-ID-PCE-2011-3 - Molecules and mechanisms involved in cytokine and chemokine-dependent vascular inflammation as targets for novel nano-therapeutic strategies, Exploratory Research Projects – REFINATER 9. 2011-2014: European Innovative RTD Projects Proposals in Nanomedicine EuroNanoMed JTC 2010, FP7,“Nanoparticles designed to target chemokine-related inflammatory processes in vascular diseases and cancer metastasis and implementation of a biosensor to diagnose these disorders” – NANODIATER |
||||||
Patents: |
Cecoltan Sergiu, Mihaela Vadana, Letitia Ciortan, Gan Ana-Maria, Tucureanu Monica Madalina, Mihaila Cristina Andreea, Elena Butoi. Procedeu de obținere a unui hidrogel din țesut cardiac funcționalizat cu un agent antiinflamator. Cerere de brevet de inventie, nr. A 100515 din 25.08.2022
Cecoltan Sergiu, Elena Butoi, Razvan Macarie, Letitia Ciortan, Mihaela Vadana, Ileana Manduteanu. Procedeu de obţinere a unui model 3D de foiţă valvulară bioprintabilă, buletinul oficial de proprietate industrială, brevete de invenţie, Nr. 5/2021.
Diana Elena Ciolacu, Anca Roxana Petrovici, Andreea Cristina Mihaila, Elena Butoi. Procedeu si compozitie pentru obtinerea unor materiale pe baza de exopolizaharide cu potentiale aplicatii in ingineria tisulara a valvelor aortice, Nr. cerere: A 2019 00866, din 05.12.2019. |
||||||