National Grants
Project financed by The Executive Unit for the Financing of Higher Education, Research, Development and Innovation (UEFISCDI)
PROGRAM: Development of the national CD system
PROJECT TYPE: Postdoctoral research (PD) projects
PROJECT TITLE: Impact of high glucose in valvular endothelial cells-monocyte crosstalk: molecular signatures and role in early valvular dysfunction
PROJECT CODE: PN-III-P1-1.1-PD-2021-0498
CONTRACT NO.: PD 35/2022
PROJECT LEADER: PhD. Monica Ţucureanu
PROJECT MENTOR: PhD. Ileana Mânduțeanu
PROJECT ACRONYM: VALDYSIGN
IMPLEMENTATION PERIOD: 01.04.2022 – 31.05.2024
BUDGET: 250.000 lei
IMPLEMENTATION TEAM: Monica Ţucureanu, Ileana Mânduțeanu
ABSTRACT. Aortic valve disease (AVD) and diabetes are progressive diseases that represent world-wide health problems. Diabetes is a risk factor for AVD, predicts faster disease progression and accelerates AVD. The mechanisms of early aortic valve dysfunction are still unclear, but evidence suggests that valvular endothelial cells (VEC) and monocytes (Mo) play key roles in the process. Our working hypothesis is that diabetic conditions (high glucose-HG) alter normal VEC-Mo interaction by progressively inducing molecular changes and function alteration in both cell types. The main goal of this project is to reveal the molecular signatures of VEC and Mo upon their interaction in HG conditions, to evaluate the role of key molecules in cell dysfunction and to propose novel mechanisms of early valvular dysfunction in diabetes. Our hypothesis will be tested by characterizing the molecular profile induced by HG in interacted VEC-Mo and by evaluating the function of relevant HG-induced molecules in endothelial permeability and monocyte adhesion/transmigration. HG-induced VEC and monocyte transcriptome will be analyzed, and key molecules will be validated at gene and protein level. The role of relevant molecules will be investigated in endothelial permeability and monocyte adhesion/transmigration, early processes of valvular dysfunction in diabetes. Our project would provide important knowledge of early AVD in diabetes and might indicate new possible therapeutic targets for AVD in diabetes.
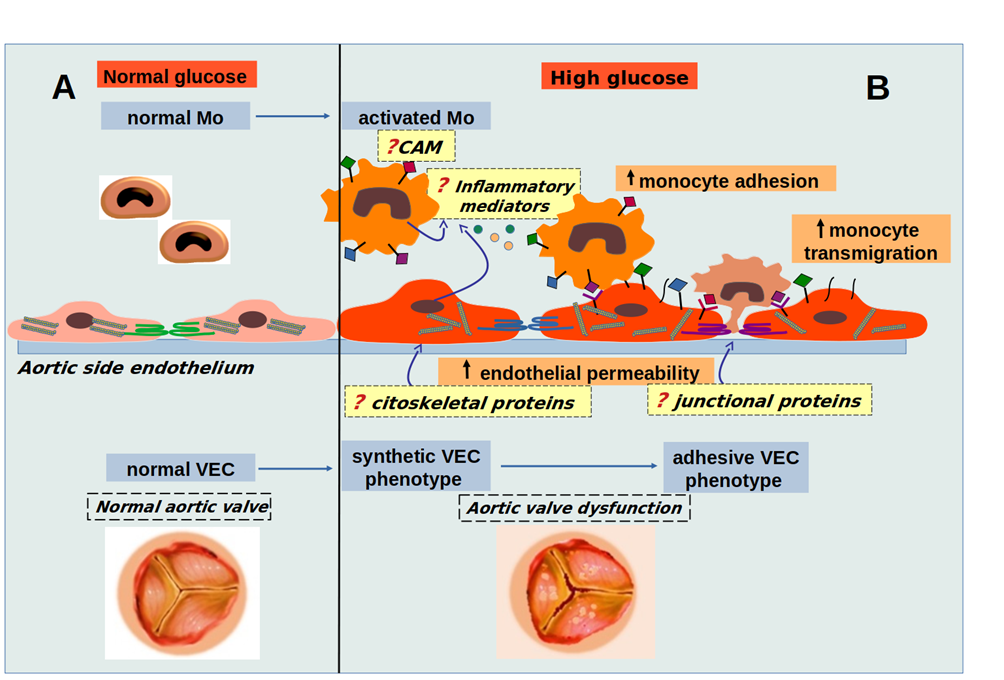
Working hypothesis. In normal glucose conditions (A), monocytes (Mo) and valvular endothelial cells (VEC) crosstalk contribute to valvular homeostasis, while in high glucose (HG) conditions (B), cells crosstalk is altered and HG progressively induces molecular changes in VEC and Mo, their interaction leading to changes in cytoskeletal and junctional proteins, thus influencing endothelial permeability, the expression of inflammatory mediators and cellular adhesion molecules (CAM), determining adhesion and transmigration of monocytes, key events in aortic valve pathology. The goal of the proposed project is to uncover molecules and mechanisms involved in early changes that lead to aortic valve dysfunction in diabetic conditions.
OBJECTIVES:
Objective 1. To characterize the molecular profile induced by short high glucose exposure in VEC-Mo co-culture.
Activity 1.1. Whole-transcriptome sequencing to identify molecules involved in early modifications induced in VEC and Mo after interaction in HG conditions;
Activity 1.2. Validation of key molecules by RealTime PCR;
Activity 1.3. Investigation of protein expression of relevant molecules induced by HG conditions in VEC-Mo co-culture.
Objective 2. To evaluate the function of relevant molecules found modified in O1 in endothelial permeability and monocyte adhesion and transmigration.
Activity 2.1. Investigation of endothelial permeability in HG conditions: role of changes in junctional and cytoskeletal proteins;
Activity 2.2. Establishing the role of diabetes on monocyte adhesion and transmigration in dynamic conditions;
Activity 2.3. Analysis of the role of identified molecules in endothelial permeability and monocyte adhesion and transmigration in dynamic conditions.
DISSEMINATION:
- Participation in the International Conference and XXXIX Annual Scientific Session of the Romanian Society of Cell Biology, 21-23 October 2022, Cluj-Napoca, Romania - MOLECULAR SIGNATURES OF VALVULAR ENDOTHELIAL CELLS AND MONOCYTES CROSS-TALK IN EARLY DIABETIC CONDITIONS. Monica Tucureanu, Letitia Ciortan, Ileana Manduteanu– poster presentation.
- Bioinformatic analysis of molecular mechanisms underlying the aortic valvular dysfunction in diabetes. Monica Tucureanu. Participation in the conference “Crossing bridges between bioinformatics and clinical research – Genetoberfest GO2023”, from 16-19 October 2023, Munich, Germany – oral presentation;
- Molecular mechanisms underlying the aortic valvular dysfunction in diabetes. Monica Tucureanu, Letitia Ciortan, Razvan Macarie, Andreea Mihaila, Elena Butoi, Ileana Manduteanu. Participation in the 44th ANNUAL SCIENTIFIC SYMPOSIUM OF THE INSTITUTE OF CELLULAR BIOLOGY AND PATHOLOGY “NICOLAE SIMIONESCU” held jointly with the 40th Annual Scientific SESSION OF THE ROMANIAN SOCIETY FOR CELL BiOLOGY, in the period 16-17 November 2023, Bucharest, Romania – poster presentation.
- The Specific Molecular Changes Induced by Diabetic Conditions in Valvular Endothelial Cells and upon Their Interactions with Monocytes Contribute to Endothelial Dysfunction. Monica Madalina Tucureanu, Letitia Ciortan, Razvan Daniel Macarie, Andreea Cristina Mihaila, Ionel Droc, Elena Butoi and Ileana Manduteanu. Int. J. Mol. Sci. 2024, 25,3048. https://doi.org/10.3390/ijms25053048 – ISI Paper.
MAIN SCIENTIFIC RESULTS:
✓ List of the modified molecules in valvular endothelial cells and interacting monocytes in diabetic conditions - the role of exposure to diabetic conditions or the interaction between VECs and monocytes in endothelial dysfunction and monocyte activation was highlighted. Genes enriched in the following KEGG pathways were identified in VECs: regulation of actin cytoskeleton (hsa04810), PI3K-Akt signaling pathway (hsa04151), focal adhesions (hsa04510), tight junctions (hsa04530), adherens junctions (hsa04520), cell adhesion molecules (CAMs) (hsa04514), and transendothelial leukocyte migration (hsa04670). In monocytes cultured in diabetic conditions or interacting with VECs in normal or high glucose conditions, genes enriched in the following KEGG pathways were expressed: extracellular matrix-receptor interaction (hsa04512), transendothelial leukocyte migration (hsa04670), cell adhesion molecules (hsa04514), TLR signaling pathway (hsa04620), NF-kB signaling pathway (hsa04064), JAK-STAT signaling pathway (hsa04630), TNF signaling pathway (hsa04668), and chemokine signaling pathway (hsa04062).
✓ Evaluation of the functional role of the molecules found to be modified in endothelial permeability and monocyte adhesion and transmigration - exposure of VECs to diabetic conditions led to the modification of key molecules and regulatory proteins, such as: increased expression of VASP and ROCK1 (involved in cytoskeletal regulation), increased expression of JAM2, cadherin-2, and integrins α4, α5, and β2 (involved in intercellular adhesion and cell-matrix interactions), and decreased expression of paxillin (an adaptive protein of focal adhesions). In addition, the interaction between VECs and monocytes in normal glucose conditions resulted in increased expression of VASP, ROCK1, β2 integrin, and PI3K activation, as well as decreased expression of proteins involved in focal adhesions (FAK and paxillin) and junctional proteins (claudin-5 and cadherin-5). Under high glucose conditions, this interaction induced PI3K signaling pathway activation, decreased expression of junctional proteins (FAK, caveolin-1, and paxillin), and increased expression of E-selectin. Furthermore, the interaction under high glucose conditions highlighted decreased expression of integrins α1, α5, αV, αVβ5, α5β1, and cadherin-2. These modifications led to cytoskeletal disorganization, changes in junctional complexes, reduced VEC adhesion to extracellular matrix proteins, increased permeability, and increased monocyte adhesion and transmigration, indicating altered valvular endothelial barrier function.
✓ Description of the mechanisms induced by increased glucose in the interaction between monocytes and valvular endothelial cells, relevant for early diabetes in the aortic valve - the ROCK and PI3K signaling pathways were identified as activated in VECs under diabetic conditions or after interaction between VECs and monocytes. The results indicate the partial involvement of these signaling pathways in cytoskeletal regulation, regulation of focal adhesion assembly or disassembly, regulation of intercellular junctions, and regulation of cell adhesion molecule expression. Furthermore, it was shown that PI3K is involved in the regulation of endothelial permeability. All of these data indicate that both ROCK and PI3K play an important role in regulating the mechanisms induced by increased glucose in the interaction between monocytes and VECs and could be potential therapeutic targets in aortic valve disease associated with diabetes.
SCIENTIFIC REPORT PHASE I 
SCIENTIFIC REPORT PHASE II 
SCIENTIFIC REPORT PHASE III 

