National Grants
Apolipoprotein E - based novel anti-atherosclerotic cell therapy approaches
“Noi proceduri terapeutice celulare anti-aterosclerotice bazate pe apolipoproteina E”
PN-II-ID-PCE-2011-3-0591”
Contract 197/2011 finantat de UEFISCDI
Cardiovascular disease is the main cause of mortality in all developed countries (55%) and atherosclerosis is the main contributor to this affliction, despite recent advances in cardiovascular medicine. This project addresses this serious disease, and its results are expected to contribute to the development of novel strategies for the treatment or prevention of atherosclerosis.
Atherosclerosis is a multifactorial process, a disorder of large and medium-size arteries characterized by progressive atherosclerotic plaques (1-5). Apolipoprotein E (apoE), a glycoprotein of 34 kDa, is a major component of the lipoprotein transport system, playing important roles in lipid metabolism (6-11). Deficiency in apoE results in atherosclerosis in humans and in animal models (12-17). ApoE mediates lipoprotein clearance from plasma, being a ligand for the LDL receptor, found on the liver and other tissues, and for LDL receptor-related protein, found on the liver (18-20). A malfunction of these mechanisms of cholesterol clearance leads to accumulation of remnants in plasma, a process associated with premature atherosclerosis (21-23). ApoE regulates plasma cholesterol levels, having also important roles in cholesterol efflux, as revealed by studies in patients and animal models with apoE deficiency or defective apoE genes (24-31). At the site of atherosclerotic lesion, apoE is provided by macrophages. We have previously showed that inflammatory conditions (similar to those found at the atherosclerotic site) lead to downregulation of the endogenous apoE in macrophages (32). Thus, despite the fact that macrophages are present in the lesion, their ability to regress atherosclerosis appears to be seriously compromised when apoE is not expressed.
Based on these findings, the central hypothesis of this proposal is that increasing local concentration of apoE at the site of atherosclerotic plaque using novel cell therapy approaches will provide significant benefits in the fight against atherosclerosis. Thus, the goal of this proposal is to use different approaches to induce an increased apoE secretion by the different cell types involved in the progression of atherosclerotic plaques. We will focus on monocytes/ macrophages and endothelial cells (EC), since they play a key role in the progression of atherosclerotic plaque.
Previous studies showed that the systemic increase of apoE expression can lead to hypertriglyceridemia (33-38), and thus the gene therapy applications to correct remnant removal disorders are compromised. In the current project, we plan to circumvent the side effects that appear in systemic increase of apoE expression by developing three strategies to augment specifically the apoE secretion at the level of lesion prone areas or in the atherosclerotic plaque, in order to prevent or regress the atherosclerotic process. All these therapeutic approaches having fewer side effects than systemic administration will open new horizons for developing cell-specific therapies using other anti-atherosclerotic molecules. These complementary therapeutic approaches are intended to meet the necessity of the patient-oriented treatment.
The goal of the project is to induce an increased apoE expression in monocytes and endothelial cells (EC) in order to prevent or regress the atherosclerotic process. For this, we plan to increase the local apoE level in the atherosclerotic plaque, in order to slow-down or regress the atherosclerotic plaque. Three main directions we be followed: (I) apoE gene regulation in macrophages; (II) monocytes transplant; and (III) a mouse model of endothelial expression of apoE, as illustrated in the Figure 1.
Figure 1. The main directions of the project.
The project has 3 objectives:
1. To identify the transcriptional mechanisms leading to activation of apoE expression. We will investigate transcriptional regulation of apoE gene in monocyte, macrophages and endothelial cell. For this we cloned the regulatory elements of apoE and their deletion fragments in a promotorless vector containing luciferase as reporter, as depicted in Fig. 2.
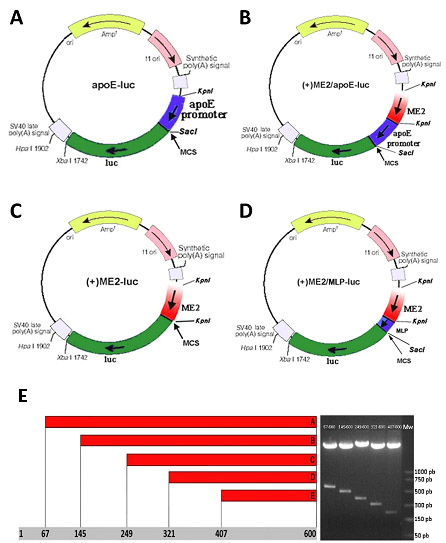
Figure. 2. ApoE regulatory elements cloned in pGL3 basic vector: -500à+73 apoE promoter (A); -500à +73 apoE promoter and ME.2. (B), ME.2 alone or in front of a mimimal promoter -MLP(C, and D respectively); ME.2 deletion fragments identified by KpnI digestion (E).
Our data revealed that apoE is upregulated by some transcription factors, among which STAT1 (Figure 3), RXR, glucocorticoids, while others strongly repress apoE gene expression.
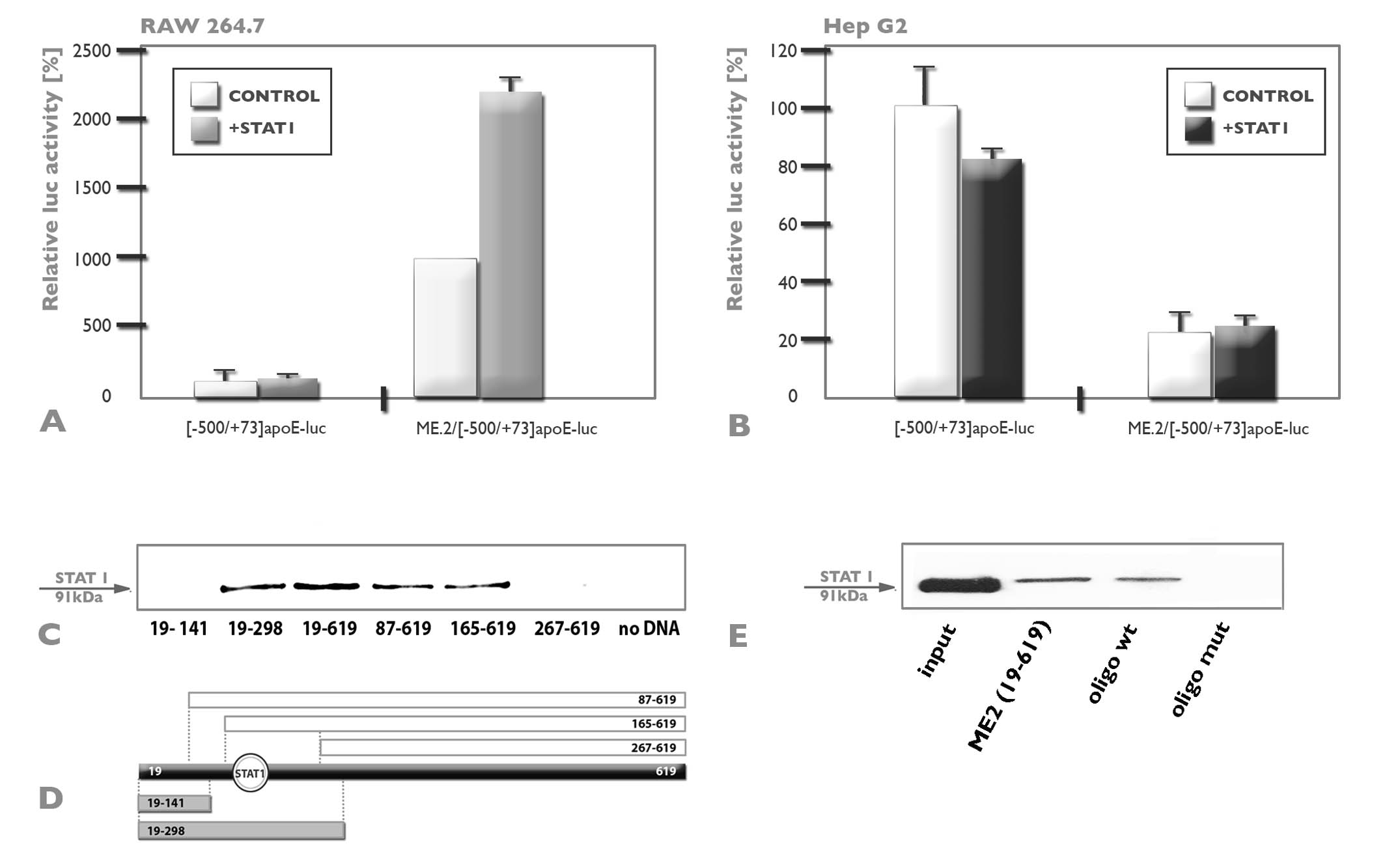
Figure 3. Transactivation of the apoE promoter in RAW 264.7 macrophages via interaction with STAT1 transcription factor acting on the multienhancer 2. Panel A, B. In RAW 264.7 cells, the overexpression of STAT1 does not increase the apoE promoter activity ([-500/+73]apoE-luc), but the activity of the apoE promoter in the presence of ME.2 (ME.2/[-500/+73]apoE-luc) is augmented by STAT1 overexpression. Overexpression of STAT1 in HepG2 cells does not increased the activity of apoE, neither in the absence, nor in the presence of ME.2. Panel C, D, E. STAT1 binding site on ME.2. DNA pull down assays was performed using different fragments of the ME.2 (schematic illustrated in Panel D), or with wild type or mutated 167-189 ME.2 region and nuclear extract obtained from RAW 264.7 cells transfected with expression vectors for STAT1. Note that the whole ME.2 sequence, as well as the following ME.2 fragments: 19-298, 87-619, 165-619 bind STAT1 transcription factor; by contrast, 19-141, as well as 267-619 fragments of ME.2 do not bind STAT1 (Panel C). These results indicate a STAT1 binding site in the region 165-267 of ME.2. STAT1 binds to the native 167-189 ME2 region (Panel E, lane oligo wt), and the binding is abrogated when the STAT1 binding site is mutated (Panel E, lane oligo mut). In the positive control, lane ME2 (19-619), the whole ME2 sequence was used (Panel E). V.G.Trusca, E.V. Fuior, I.C. Florea, D. Kardassis, M. Simionescu, A.V. Gafencu; J Biol Chem. 2011 286(16):13891-904.
In addition, we demonstrated that STAT1 and RXRα physical interact, by GST pull down assays and co-immunoprecipitations. STAT1 efficiently bound to the GST-RXRα-beads but not to the GST-beads that were used as a negative control (Figure 3 left). Co-immunoprecipitation of STAT1 and RXRα was performed using cellular extracts obtained from HEK-293T cells overexpressing both STAT1 and RXRα proteins. The results showed that STAT1 was efficiently immunoprecipitated by anti-STAT1 antibodies (Fig. 5D, lane anti-STAT1), but was also co-immunoprecipitated using anti-RXRα antibodies (Figure 3 right).
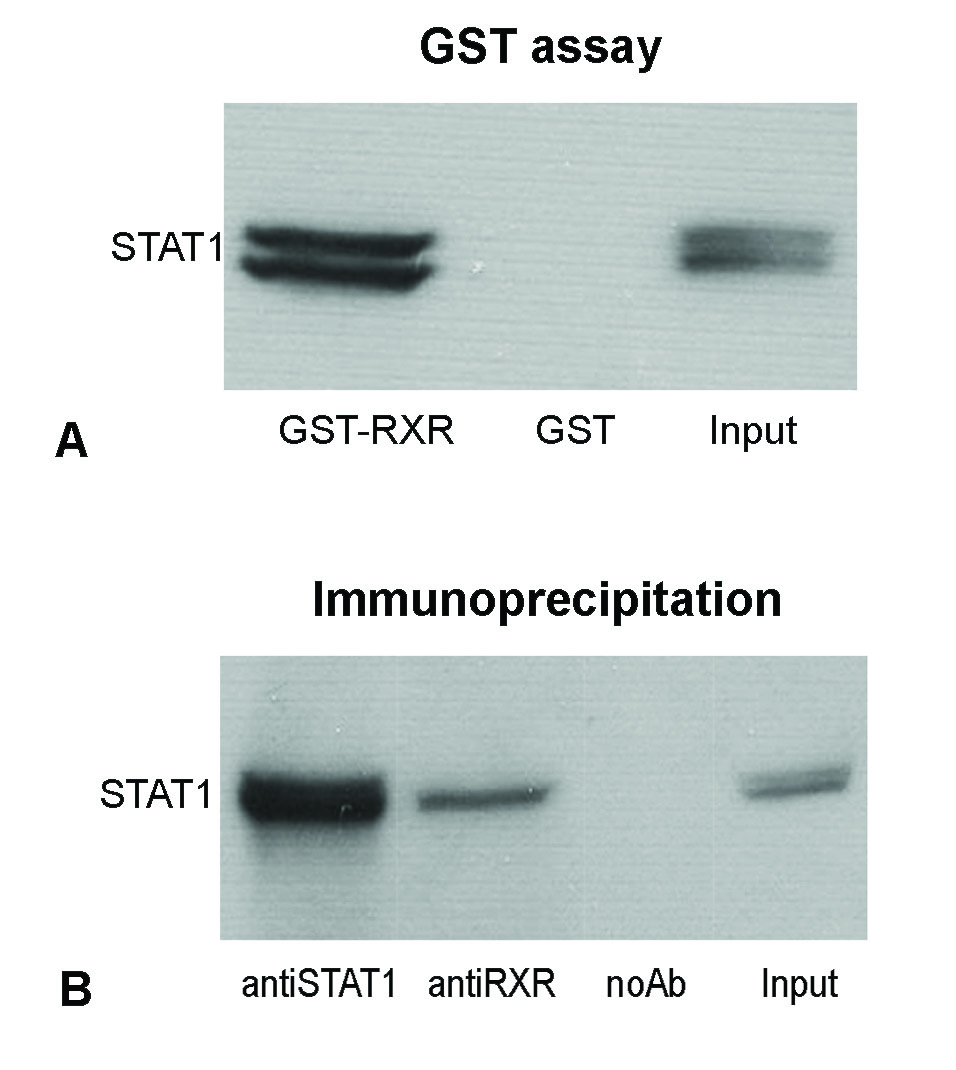
Figure. 4. STAT1 interacts with RXRα. GST-pull down assay (left) performed using cellular extracts from STAT1-overexpressing RAW 264.7 macrophages indicated that STAT1 proteins bind efficiently to the GST-RXRα-beads (lane GST-RXRα), but cannot bind to the GST beads (lane GST); Co-immunoprecipitation experiments (right) were performed using cellular extracts from HEK-293T cells overexpressing both STAT1 and RXRα. Western blotting of immunoprecipitated proteins done with anti-STAT1 antibodies revealed that STAT1 proteins were efficiently immunoprecipitated by anti-STAT1 antibodies (lane anti-STAT1), but they were also co-immunoprecipitated using anti-RXRα antibodies (lane anti-RXRα). No bands were obtained in the negative control, (lane NoAb). VG. Trusca, IC. Florea, D. Kardassis, AV. Gafencu, PLoS ONE (7) 2012.
2. To target apoE expressing monocytes at the atherosclerotic plaques.
In our approach, a natural phenomenon occurring during atherogenesis, namely monocyte infiltration in the atherosclerotic plaque (AP) would be used as a mean for AP targeting. Targeting apoE expressing monocytes at the AP may increase the apoE production in the plaque, leading to an increased cholesterol efflux, with potential anti-inflammatory and antiproliferative effects.
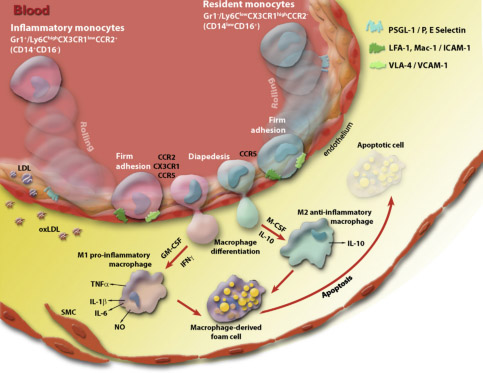
Figure 5. The involvement of the endothelium and monocytes /macrophages in chronic inflammation associated with atherosclerosis, IM Fenyo and AV Gafencu Immunobiology, 218 (11), 2013
We plan to (2a) induce ex vivo apoE expression in monocytes; (2b) test the minimal monocyte activation that allows the most efficient infiltration in atheroma; (2c) transplant apoE expressing monocytes in apoE null mice and follow plaque regression.
Data obtained so far demonstrated the infiltration of the activated monocytes in the atherosclerotic plaque of the aorta (Figure 6A) and a positive of monocytes transplant on atherogenesis. Other organs (mainly the lung) are also stained after monocyte transplant (Figure 6B). The aortic lesions sizes were determined after Oil Red O staining, as illustrated in the Figure 6C.
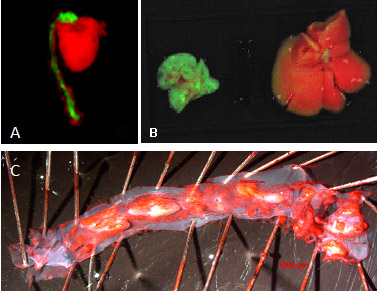
Figure 6. Macrophage infiltration in apoE-/- mouse aorta (A), lung and liber (B) – green color. Atherosclerotic plaque of the mouse aorta was revealed by Oil Red O staining (C). Dumitrescu et al., manuscript in preparation.
3. To generate a tissue specific transgenic mouse model that expresses apoE specifically in the endothelium and to assess its sensitivity to atherosclerosis. We will obtain transgenic mice with inducible endothelial-specific expression of apoE3 and evaluate the regression of the atherosclerotic plaques in mice expressing apoE3 in EC.
For this obtained and cross-breed two transgenic mice (i) carrying the reverse tetracycline transactivator expressed under an endothelial specific promoter (Tek -rtTA) in the apoE null mouse (Tek-rtTA apoE null) and (ii) carrying the apoE3 driven by the promoter provided with a modified tetracycline-responsive element, expressed also in the apoE null mouse, is depicted in the Figure 7.
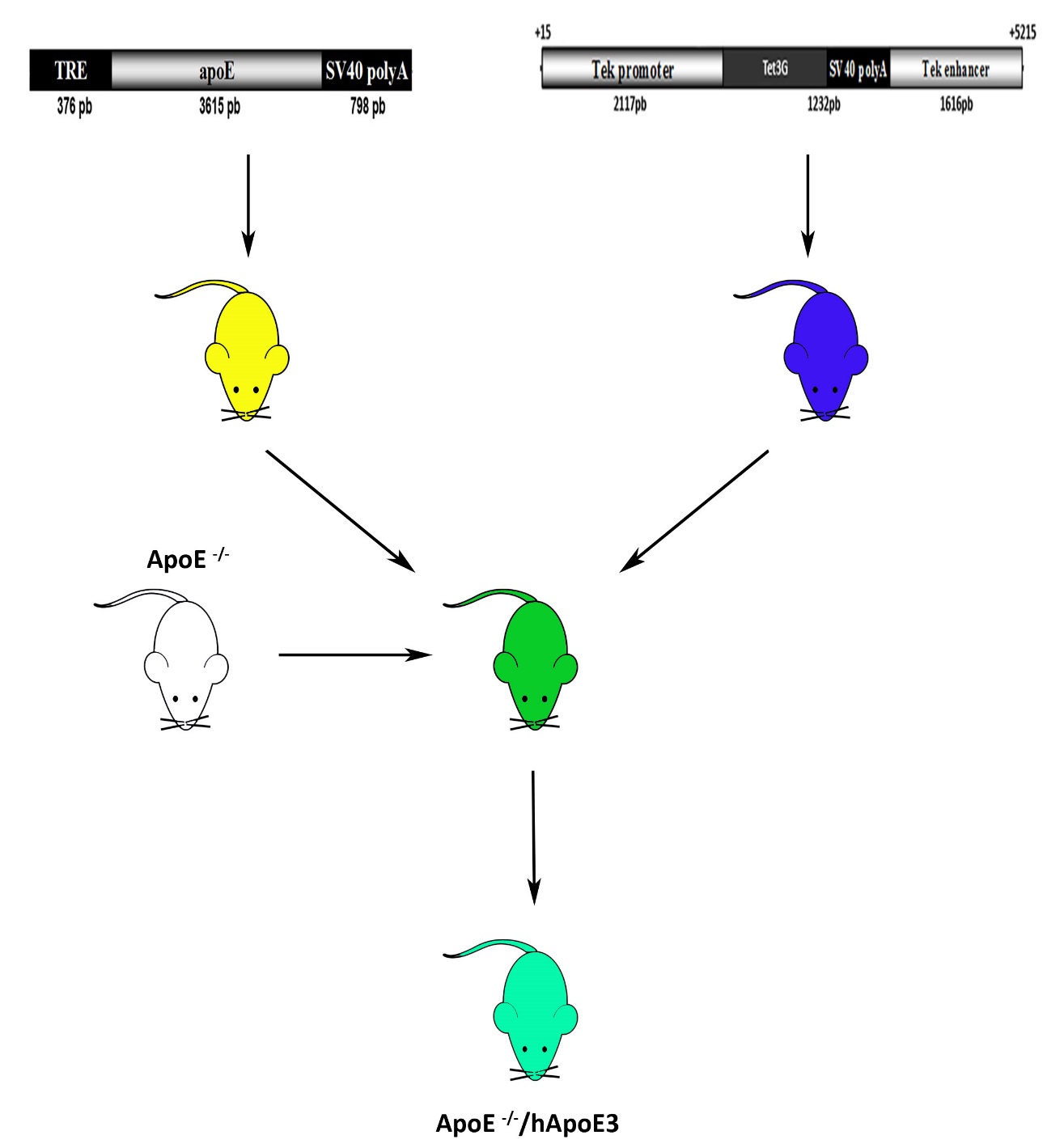
Figure 7. Schematic illustration of production of the mouse model with endothelial specific and conditional apoE expression.
In the first two stages we have obtained the constructs used for the transgenesis, and in the third stage the DNA was microinjected in the pronucleus of the zygotes. First generation of puppies was obtained. Then the mice were cross breaded as depicted in the figure 7.
The puppies were genotyped in order to detect the inserted transgene. The PCR products amplified from some TRE-apoE positive mice and from TEK-TETON are presented below:

Figure 8. An example of TRE-apoE and TEK-TETON micegenotype. Madalina Fenyo et al., manuscript in preparation
REFERENCES
- Ylä-Herttuala S., Acta Med Scand Suppl. 1985;701:7-14
- Valtonen V.V., Am Heart J. 1999;138 (5 Pt 2):S431-3
- Packard R.R. and Libby P., Clin Chem. 2008;54(1):24-38
- Ghinea N. et al., J Submicrosc Cytol. J Submicrosc Cytol. 1987;19(2):209-27
- Simionescu M., Arterioscler Thromb Vasc Biol. 2007;27(2):266-74
- Shore B. and Shore V., Biochemistry 1973;12:502-507
- Green P.H. et al., J Clin Invest. 1979;64(1):233-42
- Danielsson B. et al., FEBS Lett. 1978; 86(2):299-302
- Schaefer E.J. et al., Lipids. 1979; 14(5):511-22
- Wu A.L. et al., Am J Clin Nutr. 1980;33(3):582-9
- Innerarity T.L. et al., Biochemistry 1978;17:1440-1447
- Ghiselli, G. et al., Science 1981;214:1239-1241
- Schaefer, E.J., et al., J. Clin. Invest. 1986, 78:1206-1219
- Cladaras, C., et al., J. Biol. Chem. 1987;262:2310-2315
- Plump, A.S. et al., Cell 1992;71:343-353
- Zhang, S.H. et al., Science 1992;2588:468-471
- Reddick, R.L. et al., Arterioscler. Thromb. 1994;14:141-147
- Mahley, R.W., Innerarity, T.L. Biochim. Biophys. Acta 1983;737:197-222
- Brown, M.S., Goldstein, J.L. Science 2001; 92:34-47
- Beisiegel, U. et al., Nature 1989;341:162-164
- Schaefer, E.J., et al., J. Clin. Invest. 1986;78:1206-1219
- Zhang, S.H., et al., Science 1992;2588:468-471
- Reddick, R.L. Arterioscler. Thromb. 1994;14:141-147
- Nakashima Y. et al., Arterioscler Thromb. 1994;14:133-140
- Von Eckardstein, A. Curr. Opin. Lipidol. 1996;7:89-95
- Davignon J. Arterioscler Thromb Vasc Biol. 2005;25:267-269
- Raffai R.L., et al., Arterioscler Thromb Vasc Biol. 2005;25:436-441
- Ali K., et al., Circ Res. 2005;97:922-927
- Grainger D.J. et al., J Immunol. 2004;173:6366-6375
- Linton M.F., et al., Science. 1995;267:1034-1037
- Van Eck M. et al., Arterioscler Thromb Vasc Biol. 1997;17(11):3117-3126
- Gafencu A.V. et al., J Biol Chem. 2007;282:21776 -21785
- Huang Y. et al., J. Biol. Chem 1998;273:26388-26393
- Ehnholm C. et al., Proc. Natl.Acad. Sci. USA 1984;81:5566-5570
- Huang Y., et al., J. Biol. Chem. 1998;273:17483-17490
- Rensen, P.C.N. and van Berkel T.J.C., J. Biol. Chem. 1998;271:14791-14799
- Jong M.C. et al., Biochem. J. 1997;328:745-750
- Huang Y., et al., Arterioscler. Thromb. Vasc. Biol. 1999;19:2952-2959
Research team:
-Dr. Anca Gafencu – coordinator (anca.gafencu@icbp.ro)
-Dr. Violeta Trusca
-Dr. Madalina Fenyo
-Dr. Simona Stravri
-Dr. Madalina Dumitrescu
Publications in ISI Journals
- STAT1 INTERACTS WITH RXRa TO UPREGULATE APOCII GENE EXPRESSION IN MACROPHAGES; Violeta G. Trusca, Irina C. Florea, Dimitris Kardassis, Anca V. Gafencu, PLoS ONE (7) 2012, e40463 (Impact Factor 4.092).
- THE INVOLVEMENT OF THE MONOCYTES /MACROPHAGES IN CHRONIC INFLAMMATION ASSOCIATED WITH ATHEROSCLEROSIS, Ioana Madalina Fenyo, Anca V. Gafencu, Immunobiology, 2013, 218 (11), p1376-1384. (Impact Factor 3.205).
Manuscripts in preparation or sent for publication:
1. Beyond lipoprotein receptors: learning from mouse models about new targets for reduction of the atherosclerotic plaque; Violeta G. Trusca, et al.
2. The effect of metformin in the reversion of apoE down-regulation under the inflammatory stress; Simona Stavri, et al.
3. The role of the thyroid hormones in apoE gene regulation; Corina Roman et al.
4. ApoE expressing monocytes targeting the atherosclerotic plaques; Madalina Dumitrescu, et al.
5. A tissue specific transgenic mouse model expressesing apoE specifically in the endothelium; Madalina Fenyo et al.
Chapters in books
- MOLECULAR TARGETS FOR ATHEROSCLEROSIS TREATMENT, Ioana Madalina Fenyo, Anca V. Gafencu, From Vascular Cell Biology to Cardiovascular Medicine, ISBN 987-81-7895-503-2 Transworld research Network, 2011
- REGULATION OF HDL GENES: TRANSCRIPTIONAL, POSTTRANSCRIPTIONAL, AND POSTTRANSLATIONAL, Dimitris Kardassis, Anca Gafencu, Vassilis I. Zannis and Alberto Davalos, High Density Lipoproteins: From Biological Understanding to Clinical Exploitation, ISBN-13: 978-3319096643 ISBN-10: 3319096648 Springer, 2015
Oral presentation at international meetings
- APOE GENE REGULATION VIA LONG-RANGE INTERACTIONS IN MACROPHAGES -Violeta Trusca, Dimitris Kardassis, Anca Gafencu. 2ND WG/MC MEETING of the action COST BM 0904 “HDL: From Biological Understanding to Clinical Exploitation, January 26-27, 2012, Barcelona.
- MACROPHAGE-SPECIFIC UP-REGULATION OF APOLIPOPROTEIN E GENE EXPRESSION BY STAT1 IS ACHIEVED VIA LONG RANGE GENOMIC INTERACTIONS 80th European Atherosclerosis Society Congress Milan, Italy, May 25-28, 2012 - Violeta Trusca, D. Kardassis, M. Simionescu, A. Gafencu.
- MACROPHAGE-SPECIFIC UPREGULATION OF APOE AND APOCII GENES BY STAT1 ACTING ON THE MULTIENHANCER 2, Anca V. Gafencu, Violeta Trusca, Dimitris Kardassis, Maya Simonescu – the 26th meeting of the European Macrophage and Dendritic Cell Society, Debrecen, Hungary.
- GENERATION OF A MURINE TRANSGENIC MODEL WITH CONDITIONAL EXPRESSION OF HUMAN APOLIPOPROTEIN E3 IN THE VASCULAR ENDOTHELIUM Ioana Madalina Fenyo The 32th Annual Scientific Session of Romanian Society for Cell Biology, Targu Mures, 2014, Bulletin of Romanian Society for Cell Biology Nr.42 / 2014 , p21
Posters presented at international meetings:
- LPS DOWN-REGULATES APOCI IN MACROPHAGES, Minodora Tudor, Violeta Trusca, Ana-Maria Eftimie, Elena Fuior, Anca Gafencu, Annual session of the Romanian Society for Cell Biology, Satu Mare-Debrecen June 2012, Abstract Book, page 122.
- MACROPHAGE -SPECIFIC UPREGULATION OF APOE AND APOCII GENE BY STAT1 ACTING ON THE MULTIENHANCER Anca Gafencu, Violeta Trusca, Dimitris Kardassis, Maya Simionescu Annual session of the Romanian Society for Cell Biology, Satu Mare-Debrecen June 2012, Abstract Book, page 95.
- STAT1 INTERACTS WITH RXR TO INCREASE APOCII GENE IN MACROPHAGES, Violeta Trusca, Dimitris Kardassis, Anca Gafencu Annual session of the Romanian Society for Cell Biology, Satu Mare-Debrecen June 2012, Abstract Book page 138.
- APOE GENE REGULATION VIA LONG-RANGE INTERACTIONS IN MACROPHAGES - Violeta Georgeta Trusca, Elena Valeria Fuior, Irina Cristina Florea, Dimitris Kardassis, Anca Violeta Gafencu. EMBL Meeting„Transcription and Chromatin” Heidelberg, 25-28 August 2012 Germany.
- MACROPHAGE-SPECIFIC UPREGULATION OF APOE AND APOCII GENES BY STAT1 ACTING ON THE MULTIENHANCER 2, Anca V. Gafencu, Violeta Trusca, Dimitris Kardassis, Maya Simonescu - the 26th meeting of the European Macrophage and Dendritic Cell Society, September 1-3, 2012, Debrecen, Hungary.
- LPS DOWN-REGULATES APOCI IN MACROPHAGES, Minodora Tudor, Violeta Trusca, Ana-Maria Eftimie, Elena Fuior, Anca Gafencu, RAMSES Meeting, Bucharest, September 2012.
- MACROPHAGE -SPECIFIC UPREGULATION OF APOE AND APOCII GENE BY STAT1 ACTING ON THE MULTIENHANCER Anca Gafencu, Violeta Trusca, Dimitris Kardassis, Maya Simonescu, RAMSES Meeting, Bucharest, September 2012.
- STAT1 INTERACTS WITH RXR TO INCREASE APOCII GENE IN MACROPHAGES, Violeta Trusca, Dimitris Kardassis, Anca Gafencu; RAMSES Meeting, Bucharest, September 2012.
- "STAT1 INTERACTS WITH RXR TO INCREASE APOCII GENE IN MACROPHAGES" Trușcă VG, Kardassis D, Gafencu AV. Poster A186-The 4th EMBO Meeting, September 22-25, 2012, Nice, France, Abstract Book page 65.
- ”MACROPHAGE-SPECIFIC UPREGULATION OF APOE AND APOCII GENES BY STAT1 ACTING ON THE MULTIENHANCER 2- Trușcă VG, Kardassis D, Simionescu M, Gafencu AV.”- Poster P8, 3rd Working Group/ Management Committee Meeting COST Action BM0904, Lille, France, January 24-25, 2013, Abstract Book, page 35.
- “GENE REGULATION OF APOLIPOPROTEIN E IN MACROPHAGES” - Trușcă VG, Kardassis D, Simionescu M, Gafencu AV. 7th International Atherosclerosis Society Workshop on HDL 2014, Roma, Italia, 2014
- “MECHANISMS OF APOLIPOPROTEIN E GENE REGULATION IN MACROPHAGES” Trușcă VG, Kardassis D, Simionescu M, Gafencu AV. The 82nd European Atherosclerosis Society Meeting, Madrid, Spania, 2014, Atherosclerosis Volume 235, Issue 2, Page e133, 2014
- “THE ROLE OF GLUCOCORTICOIDS IN APOLIPOPROTEIN E GENE REGULATION” Violeta Trusca, Dimitris Kardassis, Anca Gafencu The 32th Annual Scientific Session of Romanian Society for Cell Biology, Targu Mures, 2014 Bulletin of Romanian Society for Cell Biology Nr.42 / 2014 , p95
- BRAIN SPECIFIC REGULATION OF APOLIPOPROTEIN E GENE BY NUCLEAR RECEPTORS – Roman C, Trușcă VG, Simionescu M, Gafencu AV.”The 32th Annual Scientific Session of Romanian Society for Cell Biology, Targu Mures, 2014, Bulletin of Romanian Society for Cell Biology Nr.42 / 2014 , p63
- THE EFFECT OF METFORMIN ON APOE GENE EXPRESSION IN HUMAN THP-1 MACROPHAGES Stavri S, Trușcă VG, Gafencu AV.The 32th Annual Scientific Session of Romanian Society for Cell Biology, Targu Mures, 20142014 Bulletin of Romanian Society for Cell Biology Nr.42 / 2014 , p99
Other results:
Data obtained till present are part of the PhD Thesis of two member in the research team:
- Violeta Georgeta Trusca: Thesis entitled: Apolipoprotein gene regulation – as a therapeutic tool for atherosclerosis. Public defense: June 27, 2013
- Madalina Ioana Fenyo: Thesis entitled: Molecular interventions on the pro/anti atherosclerotic protein expression for atherosclerosis treatment. Public defense: October 30, 2013
Lucrarile ISI publicate sau acceptate pentru publicare in 2015
 Stavri S, Trusca VG, Simionescu M, Gafencu AV. Metformin reduces the endotoxin-induced down-regulation of apolipoprotein E gene expression in macrophages.Biochem Biophys Res Commun. 2015 May 29;461(2):435-40. doi: 10.1016/j.bbrc.2015.04.057.
Stavri S, Trusca VG, Simionescu M, Gafencu AV. Metformin reduces the endotoxin-induced down-regulation of apolipoprotein E gene expression in macrophages.Biochem Biophys Res Commun. 2015 May 29;461(2):435-40. doi: 10.1016/j.bbrc.2015.04.057. S Stavri, M Simionescu, D Kardassis, AV Gafencu. Krüppel-like factor 4 synergizes with CREB to increase the activity of apolipoprotein E gene promoter in macrophages, Biochem Biophys Res Commun. 2015 ;468(1-2):66-72. doi: 10.1016/j.bbrc.2015.10.163.
S Stavri, M Simionescu, D Kardassis, AV Gafencu. Krüppel-like factor 4 synergizes with CREB to increase the activity of apolipoprotein E gene promoter in macrophages, Biochem Biophys Res Commun. 2015 ;468(1-2):66-72. doi: 10.1016/j.bbrc.2015.10.163. C Roman, EV Fuior, VG Trusca, D Kardassis, M Simionescu, AV Gafencu. Thyroid hormones upregulate apolipoprotein E gene expression in astrocytes Biochem Biophys Res Commun. 2015 Oct 29. 468(1-2):190-5, doi: 10.1016/j.bbrc.2015.10.132.
C Roman, EV Fuior, VG Trusca, D Kardassis, M Simionescu, AV Gafencu. Thyroid hormones upregulate apolipoprotein E gene expression in astrocytes Biochem Biophys Res Commun. 2015 Oct 29. 468(1-2):190-5, doi: 10.1016/j.bbrc.2015.10.132.- V.G.Trusca, E.V. Fuior, A.V.Gafencu, Beyond Lipoprotein Receptors: Learning from Receptor Knockouts Mouse Models about New Targets for Reduction of the Atherosclerotic Plaque, Current Molecular Medicine vol 15, No 10, p 905-931, 2015
- I.M. Fenyo, A.M. Eftimie, E.V.Fuior, A.V. Gafencu A system for in vivo endothelial-specific and conditional expression of apolipoprotein E3 – acceptat spre publicare in Romanian Biotehnological Letters.
- M. Dumitrescu, E.V. Fuior, M.Tudor and A.V.Gafencu, Cell –transplant with apolipoprotein E3 overexpressing RAW 264.7 monocytes / macrophages Induces a decrease of the atherosclerotic plaque in a mouse model, acceptat spre publicare in Romanian Biotehnological Letters.
- M. Dumitrescu, A.V.Gafencu, and E.V. Fuior Cell – New insights on apoliprotein E involvement in brain lipid homeostasis- acceptat spre publicare in Romanian Biotehnological Letters.
Lucrarile BDI publicate
 E.V. Fuior, V.G. I.M. Fenyo, A.V Gafencu The expression of translin is regulated by inflammatory stimuli in monocytes and macrophages Annals of R.S.C.B., Vol. XIX, Issue 2, 2015, pp. 33 - 40
E.V. Fuior, V.G. I.M. Fenyo, A.V Gafencu The expression of translin is regulated by inflammatory stimuli in monocytes and macrophages Annals of R.S.C.B., Vol. XIX, Issue 2, 2015, pp. 33 - 40 - E.V. Fuior, V.G. Trusca, C.Roman, A.V Gafencu Enzymatic targets in atherosclerosis, J Mol Genet Med 9:176. doi:10.4172/1747-0862.1000176
- V.G. Trusca, A. D. Mihai, E.V. Fuior, M.I. Fenyo, A.V. Gafencu, High levels of homocysteine downregulate apolipoprotein E expression via NF-kB, acceptat spre publicare la revista World Journal of Biological Chemistry
Capitole in monografii aparute in 2015
![]() D Kardassis, A Gafencu, VI Zannis, A Davalos. Regulation of HDL genes:transcriptional, posttranscriptional, and posttranslational. Handb Exp Pharmacol. 2015;224:113-79. doi:10.1007/978-3-319-09665-0_3.
D Kardassis, A Gafencu, VI Zannis, A Davalos. Regulation of HDL genes:transcriptional, posttranscriptional, and posttranslational. Handb Exp Pharmacol. 2015;224:113-79. doi:10.1007/978-3-319-09665-0_3.
Comunicari la manifestari stiintifice nationale/internationale
- Exogenous glucocorticoids upregulate apoE gene expression in a cell-specific manner, Violeta G. Trusca, Dimitris Kardassis, Anca V. Gafencu ”World Hellenic Biomedical Congress & Summer School in Medical & Biosciences Research & Management”, Atena, Mani Laconia, Grecia, 16-24 mai 2015
- KLF4 transcription factor upregulates apolipoprotein E gene expression. Simona Stavri, Anca Gafencu ”7th National Congress with International Participation and 33rd Annual Scientific Session of the Romanian Society for Cell Biology”, Baia-Mare, (Cartea de rezumate pag 120), 10-14 iunie 2015
- Macrophage specific effects of metformin on apolipoprotein E expression under the inflammatory stress Simona Stavri Violeta Trusca, Maya Simionescu, Anca Gafencu ”7th National Congress with International Participation and 33rd Annual Scientific Session of the Romanian Society for Cell Biology”, Baia-Mare, (Cartea de rezumate pag 58 ), 10-14 iunie 2015
- Glucocorticoids upregulate apolipoprotein E gene in macrophages in a cell-specific manner Violeta G. Trusca, Madalina I. Fenyo, Dimitris Kardassis, M. Simionescu, Anca V. Gafencu, Al 7-lea Congres national al SRBC cu participare internationala si a XXXIII-a Sesiune stiintifica anuala, Baia Mare, Romania (Cartea de rezumate pag 55), 10-14 iunie 2015
- Apolipoprotein E gene regulation by omeprazole activated AHR/ARNT complex Irina Grosu, Violeta Trusca, Anca Gafencu ”Al 7-lea Congres national al SRBC cu participare internationala si a XXXIII-a Sesiune Stiintifica Anuala, Baia Mare, Romania (Cartea de rezumate pag 122), 10-14 iunie 2015
- Mechanism of apolipoprotein E modulation by metformin under normal and inflammatory states Stavri Simona, Violeta Georgeta Trusca, Maya Simionescu, Anca Violeta Gafencu The 38th Scientific Meeting of the European Lipoprotein Club”, Tutzing, Germania (Cartea de rezumate, pag. 108), 7-10 septembrie 2015
- Glucocorticoids upregulate apolipoprotein E gene expression in a cell specific manner Trusca VG, Fuior EV, Fenyo IM, Kardassis D, Simionescu M, Gafencu AV The 38th Scientific Meeting of the European Lipoprotein Club”, Tutzing, Germania, 7-10 septembrie 2015
- Cell-specific effect of glucocorticoids on apolipoprotein E gene regulation, Trusca VG, Fuior EV, Fenyo IM, Kardassis D, Simionescu M, Gafencu AV , 4th International Symposium on Adipobiology And Adipopharmacology (ISAA), Romanian Academy, Bucharest, Romania, 28-31 October 2015; abstract publicat in Romanian Journal of Diabetes, Nutrition and Metabolic Diseases, Vol. 22, Supliment 2, 2015, pag. 29
- Estrogen hormones modulate apolipoprotein E expression in astrocytes, Corina Roman, Maya Simionescu, Anca Gafencu, Al 7-lea Congres national al SRBC cu participare internationala si a XXXIII-a Sesiune Stiintifica Anuala, Baia Mare, Romania (Cartea de rezumate, pag 119), 10-14 iunie 2015
- Macrophage-specific upregulation of apolipoprotein E gene by glucocorticoids, Violeta G. Trusca, Madalina I. Fenyo, Elena V. Fuior, Dimitris Kardassis, Maya Simionescu, Anca V. Gafencu ”Chromatin biology: past, present & future” organizata de ”London Chromatin Club”, Londra, Anglia, 8-10 aprilie 2015
- Generarea unui model transgenic murin pentru exprimarea condiţională, specific endotelială, a apolipoproteinei E3 umane, Ioana Mădălina Fenyo Sesiunea anuala institutului “Victor Babeş” si al 8-lea simpozion naţional de patologie medicina de precizie - de la modele experimentale la biomarkeri, 19-21 noiembrie 2015.

