National Grants
Project title: INVESTIGATION OF MOLECULAR MECHANISMS OF ENDOTHELIN SYSTEM IN DIABETES; DEVELOPMENT OF NEW PHARMACOLOGICAL STRATEGIES TO IMPROVE VASCULAR FUNCTION
Titlul proiectului: Investigarea mecanismelor moleculare implicate in reglarea sistemului endotelinei in diabet; dezvoltarea de noi strategii farmacologice pentru imbunatatirea functiei vasculare
Project code: PN-II-RU-TE-2011-3-0142; Contract number: TE 26/2011
Contracting authority: Ministry of National Education - Executive Agency for Higher Education, Research, Development and Innovation(MEN-UEFISCDI)
Contractor:Institute of Cellular Biology and Pathology “Nicolae Simionescu”
Project director: Simona-Adriana Manea, Ph.D., Molecular and Cellular Pharmacology - Functional Genomics Laboratory, Institute of Cellular Biology and Pathology ‘Nicolae Simionescu’, Bucharest, Romania (e-mail: simona.manea@icbp.ro)
Research team:
- Constantina Heltianu, Ph.D.
- Adrian Manea, Ph.D.
- Andra Todirita, Ph.D. Student
- Alina Constantin, Ph.D.
- Nicoleta Alexandru, Ph.D.
- Florentina Safciuc, Ph.D.
Summary of the project
Diabetes mellitus represents an important risk factor for cardiovascular diseases, namely accelerated atherosclerosis and its down-stream complications - the main cause of deaths in diabetic patients. Endothelin system contributes by its components in the regulation of key physiological processes, such as vascular tonus, cellular growth and proliferation. In diabetes, dysfunctional endothelin system, which is generally characterized by excessive expression and activities of bioactive peptides (endothelins), associated receptors, and converting enzymes, induces a chain of critical processes that predispose to atherosclerotic plaque formation and vulnerability. Therefore, understanding of the molecular control mechanisms responsible for endothelin system alteration may lead to a more focused therapy of diabetes-associated cardiovascular disorders. The main aims of this research proposal is to investigate mechanisms responsible for the regulation of endothelin system constituents’ expression and function in diabetes and to counteract the ETs-induced vascular deleterious effects by pharmacological targeting of the newly identified regulators of the system. The translation into clinic of the results produced by these studies may lead to the development of novel therapeutic strategies to improve vascular function and to prevent the escalation of cardiovascular diseases and their downstream complications in the diabetic population.
Project goals
Population studies indicate that diabetes mellitus (DM), a major risk factor for cardiovascular diseases, attained an epidemic scale worldwide. DM contributes to the development of vascular disorders that ultimately affect the function of the heart, kidneys, eyes, and the nervous system (1). Accelerated atherosclerosis represents one major vascular complication of DM and is responsible for 70-80% of deaths in diabetic patients. Atherosclerosis is generally viewed as the outcome of a metabolic disorder and a chronic inflammatory reaction of large- and medium-sized arteries. It is characterized by a progressive lipid accumulation in the vessel’s intima, dysfunctions of endothelial cells (ECs) and smooth muscle cells (SMCs), and a strong contribution of infiltrated immune cells (2; 3).
Hyperglycemia, the primary clinical manifestation of diabetes, contributes to diabetic complications by inducing vascular inflammation, oxidative stress, impaired vascular relaxation, changing vascular cell metabolism, altering the vascular matrix molecules, and circulating proteins/lipoproteins (glycated, oxidated, and advanced glycation-end products (AGEs) modified molecules) (4). Nevertheless, the precise mechanisms of hyperglycemia and its associated pathological outcomes (i.e. hyperlipidemia and hyperinsulinemia) and the molecular nature of its down-stream pathophysiological effectors are still debatable issues.
Convincing evidence exists, that endothelin system plays an important role in the pathophysiology of diabetes-associated cardiovascular diseases, including atherosclerosis. Endothelin system comprises endothelins (ET1-3), endothelin converting enzymes (ECE1/2), and specific cellular receptors (ETA, ETB, ETC). Analysis of human genomic DNA revealed the existence of three distinct ET genes EDN1, EDN2, EDN3 that encode three different mature ET isopeptides, designated ET-1, ET-2, and ET-3. The ET-1 and ET-2 are both strong vasoconstrictors, whereas ET-3 is a potentially weaker vasoconstrictor compared to the other two isoforms. Two distinct ECEs (ECE-1 and ECE-2) have been cloned and sequenced. ECE-1 is predominantly expressed on the cell surface, whereas ECE-2 is located intracellularly in close proximity to the trans-Golgi network. At present, four different isoforms of human ECE-1 (ECE-1a, ECE-1b, ECE-1c, and ECE-1d) have been identified (5). The ETA receptor has a greater affinity for ET-1 than ET-2, and a greater affinity for ET-2 than ET-3, while the ETB receptor has shown equal affinities for all three endothelins. In 1993, Karne et al. have cloned a third receptor (named ETC) with slightly higher affinity for ET-3 compared to ET-1 (6).
Endothelins regulate important physiological processes including vascular tonus, cellular growth and proliferation. ETA receptors are found predominantly localized on the plasma membrane in vascular smooth muscle cells and mediate vasoconstriction and cell proliferation (7). ETB receptors are found in endothelial cells and have a dual function causing both vasoconstriction and vasodilation. Activation of the ETB receptor can cause an endothelium-dependent vasodilatation primarily through the release of NO, however it has been also demonstrated that the ETB receptor can mediate the ET-1-induced vasoconstriction.
In diabetes, endothelial dysfunction, which is generally characterized by excessive expression and activities of endothelins (especially ET-1- the main effector of the system), receptors (1), and associated converting enzymes (8) triggers a chain of critical events comprising oxidative stress, inflammation, fibrosis, and cellular phenotypic alterations of the vascular resident cells and infiltrated immune cells which converges to vascular dysfunction and ultimately cardiovascular diseases and downstream complications (9; 10). ET-1 induced oxidative stress predispose to atherosclerotic plaque development/rupture. However, the exact mechanisms of reactive oxygen species (ROS) production and the nature of ROS-generating sources are barely elucidated. In a previous study, we have demonstrated that an AP-1 dependent pro-inflammatory signalling pathway is responsible for the up-regulation of NADPH oxidase (a major inducer of oxidative stress), expression and activity in human aortic SMCs (11). Since, ET-1 is an important activator of AP-1 transcription factors, we can hypothesize that the pro-oxidant effect of ET-1 is mediated by activated NADPH oxidase. Consequently, in this project we will investigate the role of oxidative stress induced by ET-1 in vascular cells in diabetes.
Thus far, the pathophysiological processes involved in the alteration of endothelin system expression and function are insufficiently elucidated. In a recent study, we have demonstrated that the pro-inflammatory signalling pathway, Janus kinase/signal transducer and activator of transcription (Jak/STAT), mediates the up-regulation of ET-1 biosynthesis in human ECs exposed to high glucose concentration (12). Since inflammation and growth-promoting transcription factors NF-kB (13) and AP-1 (14) are also key regulators of ET-1 expression, we assume that a complex interplay among multiple transcription factors, co-activators, and/or co-repressors are coordinately involved in the up-regulation of ET-1 in diabetes and its associated cardiovascular complications. To certify this hypothesis, one of the main topics of this research project is to elucidate the complex interactions among different protein kinses/phosphateses and pro-inflammatory transcription factors to control the endothelin system members expression and activity. Because these signaling molecules are important transducers of numerous diabetes-associated cardiovascular risk factors, modulation of the upstream regulators of ET system constituents or direct pharmacological targeting of the components with aberrant expression/function, could represent a novel and efficient pharmacological strategy to attenuate the pathological effects of ETs in diabetes. In addition, it has been demonstrated that in diabetes the up-regulation of ET receptors (1) and converting enzymes (8) augments the pathophysiological effects of the systems’ bioactive peptides. However, the precise molecular mechanisms responsible for increased expression and responsiveness of ET receptors are not totally deciphered. In this context, the main goals of this research proposal are: (i) To investigate the molecular mechanisms responsible for the regulation of endothelin system constituents expression and function in diabetes; (ii) Pharmacological targeting of the newly identified up-stream regulators of endothelin system members in order to counteract the ETs-induced vascular deleterious effects in diabetes (see Figure). We assume that the experiments to be performed in vivo and in vitro in this projectare expected to elucidate the molecular mechanisms implicated in the regulation of endothelin system and bring newfindings that may provide either novel or additional means for the therapeutic management of diabetes-associated vascular diseases.
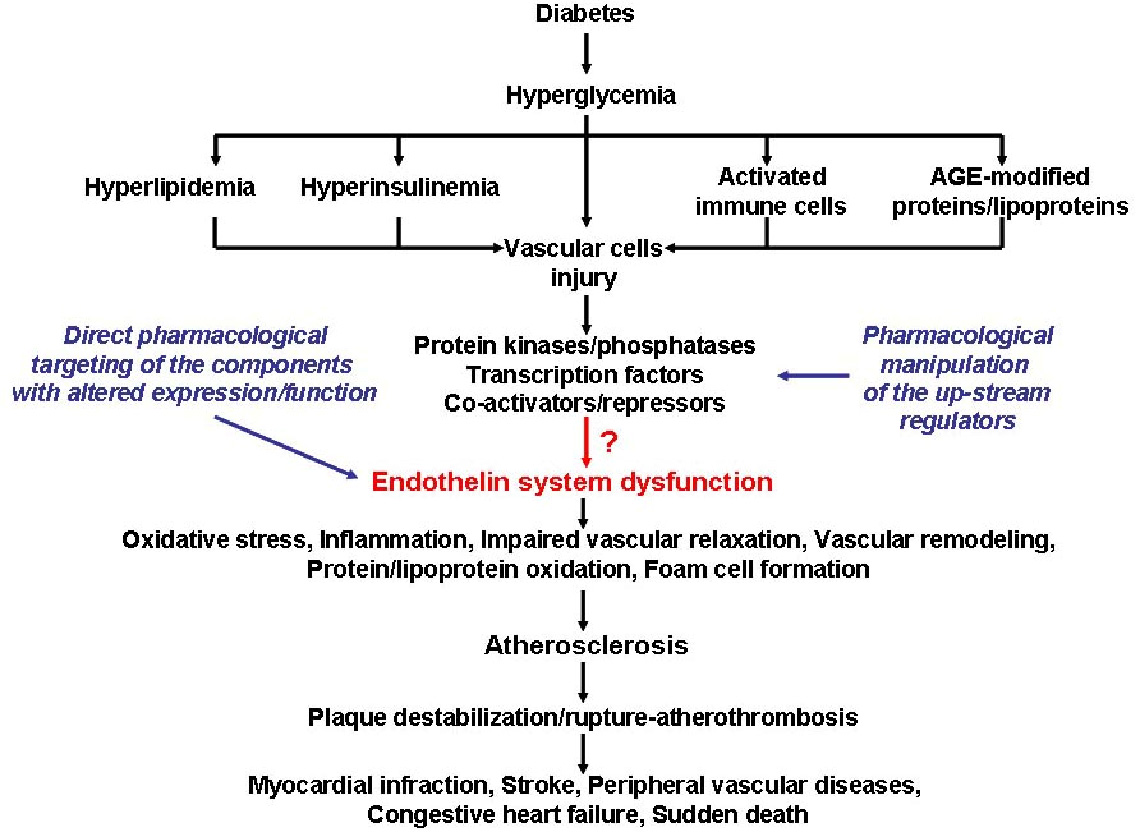
Figure legend: Schematic representation of the major goals of the research proposal.In diabetes, hyperglycemia induces severe metabolic dysfunctions (i.e., hyperlipidemia, hyperinsulinemia), and structural/functional alterations of the circulating immune cells and plasma proteins/lipoproteins. These alterations affect the vascular wall cells and activate various signaling pathways which promote endothelin system dysfunction by yet, not totally identified mechanisms. Persistent endothelin system dysfunction leads to cellular and molecular processes that favor the development of atherosclerotic lesions and down-stream complications. The diagram illustrates the possible molecular mechanisms linked to endothelin system hyperactivity in diabetes and potential pharmacological targets intended be used to control endothelin system constituents expression and activity (bleu text).
References
1. Ergul, A. (2011). Endothelin-1 and diabetic complications: Focus on the vasculature, Pharmacological Research doi:10.1016/j.phrs.2011.01.012.
2. Potenza, M.A., Addabbo, F., Montagnani, M. (2009). Vascular actions of insulin with implications for endothelial dysfunction,American Journal of Physiology - Endocrinology and Metabolism 297: E568–E577.
3. Simionescu, M. (2007). Implications of early structural-functional changes in the endothelium for vascular disease, Arteriosclerosis, Thrombosis, and Vascular Biology 27(2): 266-274.
4. Tawfik, A., Jin, L., Banes-Berceli, A.K., Caldwell, R.B., Ogbi, S., Shirley, A., Barber, D., Catravas, J.D., Stern, D.M., Fulton, D., Caldwell, R.W., Marrero, M.B. (2005). Hyperglycemia and reactive oxygen species mediate apoptosis in aortic endothelial cells through Janus kinase 2, Vascular Pharmacology 43: 320–326.
5. Lam, H.C., Lee, J.K., Lu, C.C., Chu, C.H., Chuang, M.J., Wang, M.C. (2003). Role of endothelin in diabetic retinopathy, Current Vascular Pharmacology 1(3): 243-250.
6. Karne, S., Jayawickreme, C.K., Lerner, M.R. (1993). Cloning and characterization of an endothelin-3 specific receptor (ETC receptor) from Xenopus laevis dermal melanophores, Journal of Biological Chemistry 268: 19126-19133.
7. Seo, B., Oemar, B.S., Siebenmann, R., von Segesser, L., Luscher, T.F. (1994). Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels, Circulation 89: 1203–1208.
8. Khamaisi, M., Dahan, R., Hamed, S., Abassi, Z., Heyman, S.N., Raz, I. (2009). Role of protein kinase C in the expression of endothelin converting enzyme-1, Endocrinology 150(3): 1440-1449.
9. Kalani, M. (2008). The importance of endothelin-1 for microvascular dysfunction in diabetes, Vascular Health and Risk Management 4(5): 1061–1068.
10. Potenza, M.A., Gagliardi, S., Nacci, C., Carratu, M.R., Montagnani, M. (2009). Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets, Current Medicinal Chemistry 16: 94-112.
11. Manea, A., Manea, S.A., Gafencu, A.V., Raicu,M., Simionescu. M. (2008). AP-1-dependent transcriptional regulation of NADPH oxidase in human aortic smooth muscle cells. Role of p22 phox subunit,Arteriosclerosis, Thrombosis, and Vascular Biology, 28(5): 878-885.
12. Manea, S.A., Manea, A. Heltianu C. (2010). Inhibition of the JAK/STAT signaling pathway prevents high glucose-induced increase in endothelin-1 synthesis in human endothelial cells, Cell and Tissue Research 340(1): 71-79.
13. Quehenberger, P., Bierhaus, A., Fasching, P., Muellner, C., Klevesath, M., Hong, M., Stier, G., Sattler, M., Schleicher, E., Speiser, W., Nawroth, P.P. (2000).Endothelin-1 transcription is controlled by nuclear factor-kappaB in AGE-stimulated cultured endothelial cells, Diabetes 49: 1561-1570.
14. Matsumoto, T., Kobayashi, T., Kamata, K. (2008). Relationships among ET-1, PPARγ, oxidative stress and endothelial dysfunction in diabetic animals, Journal of Smooth Muscle Research44: 41–55.
Scientific objectives
1. Evaluation of endothelin system status in cultured human aortic ECs and SMCs exposed to diabetic conditions.
2. Analysis of functional and structural alterations induced by ET-1, the main effector of the system, in ECs and SMCs.
3. Investigation of the up-stream signalling regulators of endothelin system in ECs and SMCs.
4. Investigation of endothelin system expression pattern in vascular wall cellsin diabetes in vitro and in vivo.
5. In vitro testing of the pharmacological procedures for the manipulation of the signalling pathways linked to endothelin system expression/function in ECs and SMCs.
6. Evaluation of vascular functional and structural aspects in diabetic animal models treated with pharmacological compounds directed to the up-stream regulators of the expression and function of the ET system constituents.
Expected results
Expected results 2011-2012:
• At least one study (oral presentation/poster) presented at a national/international scientific events.
• A book chapter published at an international publishing house.
Expected results 2013:
• At least one study (oral presentation/poster) presented at a national/international scientific events.
• An original article published in a journal indexed in international databases.
• An original article submitted for publication in a journal indexed by ISI.
Expected results 2014:
• At least one study (oral presentation/poster) presented at a national/international scientific events.
• An accepted original article in a journal indexed by ISI.
• An original article submitted for publication in a journal indexed by ISI.
Scientific report – brief description of the main achievements
Results
- High glucose-induced endothelial dysfunction is partially mediated by the down-stream pathophysiological effects triggered by increased expression of endothelin-1 (ET-1). The molecular control mechanisms of ET-1 synthesis are yet to be discovered. Members of the CCAAT/enhancer-binding proteins (C/EBP) family are important regulators of key metabolic processes, cellular differentiation and proinflammatory genes. In this study, we aimed at elucidating the role of C/EBP in mediating the high glucose effect on ET-1 expression in human endothelial cells (EC). Human umbilical vein cells (EAhy926) and primary cultures of human aortic EC were exposed to high levels of glucose (16.5–25 mM). Real-time PCR, Western blot, enzyme-linked immunosorbent assay, ET-1 promoter-luciferase reporter analysis, and chromatin immunoprecipitation assays were employed to investigate ET-1 regulation. High glucose activated C/EBPa, C/EBPb, and C/EBPd in a dosedependent manner. It also promoted significant increases in ET-1 gene and peptide expression. Chemical inhibition of JNK, p38MAPK and ERK1/2 diminished significantly the high glucose-induced nuclear translocation of C/EBP and ET-1 expression. Silencing of C/EBPa, C/EBPb or C/EBPd greatly reduced the high glucose-induced upregulation of ET-1 mRNA, pre-pro-ET-1, and ET-1 secretion. The expression of various C/EBP isoforms was selectively downregulated by siRNA-mediated gene silencing. In silico analysis indicated the existence of typical C/EBP elements within human ET-1 gene promoter. Transient overexpression of C/EBPa, C/EBPb or C/EBPd upregulated the luciferase level controlled by the ET-1 gene promoter. The direct interaction of C/EBPa, C/EBPb or C/EBPd proteins with the ET-1 promoter in high glucose-exposed EC was confirmed by chromatin immunoprecipitation assay. High glucose-induced ET-1 expression is mediated through multiple mechanisms. We present evidence that members of the C/EBP proinflammatory transcription factors are important regulators of ET-1 in high glucose-exposed human endothelial cells. High glucose-induced activation of C/EBP-related signaling pathways may induce excessive ET-1 synthesis, thus promoting vasoconstriction and dysfunction of the vascular wall cells in diabetes (published in PLoS One, 8 (12):e84170, 2013, Impact Factor: 3.73).
- In atherosclerosis, oxidative stress-induced vascular smooth muscle cells (SMCs) dysfunction is partially mediated by upregulated NADPH oxidase (Nox); the mechanisms of enzyme regulation are not entirely defined. CCAAT/enhancer-binding proteins (C/EBP) regulate cellular proliferation and differentiation, and the expression of many inflammatory and immune genes.We aimed at elucidating the role of C/EBP in the regulation of Nox in SMCs exposed to proinflammatory conditions.Human aortic SMCs were treated with interferon gamma (IFNγ) for up to 24h. Lucigenin-enhanced chemiluminescence, real-time PCR, Western blot, promoter-luciferase reporter analysis, and chromatin immunoprecipitation assays were employed to investigate Nox regulation. IFNγ dose-dependently induced Nox activity and expression, nuclear translocation and upregulation of C/EBPα, C/EBPβ, and C/EBPδ protein expression levels. Silencing of C/EBPα, C/EBPβ or C/EBPδ reduced significantly but differentially the IFNγ-induced upregulation of Nox activity, gene, and protein expression. In silico analysis indicated the existence of typical C/EBP sites within Nox1, Nox4, and Nox5 promoters. Transient overexpression of C/EBPα, C/EBPβ or C/EBPδ enhanced the luciferase level directed by the promoters of the Nox subtypes. Chromatin immunoprecipitation demonstrated the physical interaction of C/EBPα, C/EBPβ, and C/EBPδ proteins with the Nox1/4/5 promoters. C/EBP transcription factors are important regulators of Nox enzymes in IFNγ-exposed SMCs. Activation of C/EBP may induce excessive Nox-derived reactive oxygen species formation, further contributing to SMCs dysfunction and atherosclerotic plaque development. Pharmacological targeting of C/EBP-related signalling pathways may be used to counteract the adverse effects of oxidative stress (published in Journal of Cellular and Molecular Medicine 18(7), pg. 1467-1477, 2014, Impact Factor: 4.014).
- Endothelin system plays an important role in the pathophysiology of cardiovascular diseases. Endothelin-1 (ET-1), the main effector of the system, regulates important physiological processes including vascular tonus, cellular growth and proliferation. In pathological conditions such as diabetes, hypertension, obesity and atherosclerosis, excessive ET-1 synthesis induces oxidative stress, inflammation, fibrosis, and cellular phenotypic alterations of the vascular resident cells and infiltrated immune cells. We design this study to investigate the molecular mechanisms whereby ET-1 induces reactive oxygen species (ROS) formation in human aortic smooth muscle cells (SMC). Lucigenin-enhanced chemiluminescence and dichlorofluorescin assays indicated that exposure of SMC to ET-1 up-regulates NADPH oxidases (Nox) activity and intracellular production of ROS in a dose-dependent manner. The chemical inhibition of endothelin receptor type A (ETA) receptor, but not endothelin receptor type B (ETB), significantly reduced the ET-1-induced Nox activity. Collectively, these evidence provide new mechanistically means by which ROS are generated in SMC in response to ET-1 stimulation. Pharmacological inhibition of ETA receptor may represent a reliable therapeutic strategy to reduce oxidative stress in cardiovascular pathologies (published in Annals of RSCB, XVIII (1), 44-49, 2013).
Project Publications
Peer-review publications in ISI journals:
1. Simona-Adriana Manea, Andra Todirita, Adrian Manea. High glucose-induced increased expression of endothelin-1 in human endothelial cells is mediated by activated CCAAT/enhancer-binding proteins, PLoS One, 8 (12):e84170, 2013, Impact Factor: 3.73.
2. Simona-Adriana Manea, Andra Todirita, Monica Raicu, Adrian Manea. C/EBP transcription factors regulate NADPH oxidase in human aortic smooth muscle cells, Journal of Cellular and Molecular Medicine 18(7), pg. 1467-1477, 2014, Impact Factor: 4.014.
3. Adrian Manea, Simona-Adriana Manea, Andra Todirita, Irina Cristina Albulescu, Monica Raicu, Shlomo Sasson, Maya Simionescu. High glucose-increased expression and activation of NADPH oxidase in human vascular smooth muscle cells is mediated by 4-hydroxynonenal-activated PPARα and PPARβ/δ, Cell and Tissue Research, 361(2), 593-604, 2015, Impact Factor: 3.565.
4. Adrian Manea, Simona-Adriana Manea, Ana Maria Gan, Alina Constantin, Ioana Madalina Fenyo, Monica Raicu, Horia Muresian, Maya Simionescu. Human monocytes and macrophages express NADPH oxidase 5; a potential source of reactive oxygen species in atherosclerosis, Biochemical and Biophysical Research Communications, 461(1), 172-179, 2015, Impact Factor: 2.297.
5. Simona-Adriana Manea, Alina Constantin, Gina Manda, Shlomo Sasson, Adrian Manea. Regulation of Nox enzymes expression in vascular pathophysiology: Focusing on transcription factors and epigenetic mechanisms, Redox Biology, 5, 358-366, 2015.
Peer-review article indexed in international database:
1. Andra Todirita, Adrian Manea, Simona-Adriana Manea. Endothelin type A receptor mediates endothelin-1-induced upregulation of NADPH oxidase activity in human aortic smooth muscle cells. Annals of RSCB, XVIII (1), 44-49, 2013.
Chapter in book:
1. Constantina Heltianu, Cristian Guja, Simona-Adriana Manea. Genetic determinants of microvascular complications in type 1 diabetes. Book edited by Assoc. Prof. David Wagner, Type 1 Diabetes Complications, InTech Open Access Publisher, New York, USA, ISBN: 978-953-307-788-8, 3-28, 2011.
Oral presentation at international meetings:
1. Simona-Adriana Manea, Irina Cristina Albulescu, Catalina Luca, Adrian Manea. Positive regulation of NADPH oxidase Nox5 by pro-inflammatory mechanisms in human aortic smooth muscle cells. The 3rd EMBO Conference Series on Cellular Signaling & Molecular Medicine. Book of Abstracts. 2012.
2. Adrian Manea, Irina Cristina Albulescu, Simona-Adriana Manea, Andra Todirita, Monica Raicu. Role of peroxisome proliferator-activated receptors in mediating 4-hydroxynonenal-induced up-regulation of NADPH oxidase in human aortic smooth muscle cells. The 3rd EMBO Conference Series on Cellular Signaling & Molecular Medicine. Book of Abstracts. 2012.
3. Adrian Manea, Andra Todirita, Simona-Adriana Manea. CCAAT/enhancer-binding proteins mediate interferon gamma-induced upregulation of NADPH oxidase in human aortic smooth muscle cells. European Society of Cardiology Congress 2013. European Heart Journal, 34 (Abstract Supplement), 2013, 509.
4. Adrian Manea, Simona-Adriana Manea, Ioana Madalina Fenyo, Mihaela Loredana Antonescu, Monica Raicu, Maya Simionescu.Pharmacological inhibition of histone deacetylase reduces oxidative stress and inflammation in the aorta of diabetic mice. The 4th International Symposium on Adipobiology and Adipopharmacology (ISAA), 28-31 October 2015, Bucharest. Romanian Journal of Diabetes, Nutrition and Metabolic Diseases, Volume 22 (2015)/Supplement 2, pg. 29.
Posters presented at international meetings:
1. Simona-Adriana Manea, Alexandra Robciuc, Cristian Guja, Constantina Heltianu. Gene variants of NO/endothelin/renin-angiotensin system confer risk and protection to microangiopathy in type 2 diabetic obese subjects. 22nd European Meeting on Hypertension and Cardiovascular Protection. Journal of Hypertension, Vol 30, e-Supplement A, April 2012, e450-e451.
2. Andra Todirita, Irina Cristina Albulescu, Simona-Adriana Manea, Monica Raicu, Shlomo Sasson, Adrian Manea, Maya Simionescu. PPARα and PPARβ/δ control 4-hydroxynonenal-induced upregulation of NADPH oxidase in human aortic smooth muscle cells. 4th International Congress and 30th Annual Scientific Session of RSCB, 2012. Bulletin of Romanian Society for Cell Biology, no. 40, pg. 87.
3. Simona-Adriana Manea, Adrian Manea, Constantina Heltianu. Jak/STAT signaling pathway mediates high glucose-induced up-regulation of endothelin-1 expression in human endothelial cells. 22nd IUBMB & 37th FEBS Congress. FEBS Journal 279 (Suppl. 1), 2012, pg. 604, P28-14.
4. Simona-Adriana Manea, Andra Todirita, Adrian Manea. CCAAT/enhancer-binding proteins mediate high glucose-induced upregulation of endothelin-1 in human endothelial cells. 38th FEBS Congress “Mechanisms in Biology”. FEBS Journal 280 (Suppl. 1), 2013, pg. 297.
5. Simona-Adriana Manea, Andra Todirita, Adrian Manea. High glucose-induced increased expression of endothelin-1 in human endothelial cells is mediated by activated CCAAT/enhancer-binding proteins. The 5th International Congress and the Annual Scientific Session of Romanian Society for Cell Biology, 2013. Bulletin of Romanian Society for Cell Biology, no. 41, pg. 145.
6. Alina Constantin, Madalina Dumitrescu, Gabriela Costache, Doina Popov. Leptin induces endothelial dysfunction in high glucose conditions through mitochondrial ROS pathways. 5th International Congress and the 31st Annual Session of the Romanian Society for Cell Biology, 2013. Bulletin of Romanian Society for Cell Biology, no. 41, pg. 118.
7. Simona-Adriana Manea, Andra Todirita, Constantina Heltianu, Adrian Manea. High glucose-induced increased expression of endothelin-1 in human endothelial cells is mediated by activated CCAAT/enhancer-binding proteins. European Society of Cardiology Congress, 2013. European Heart Journal, 34 (Abstract Supplement), pg. 112.
8. Simona-Adriana Manea, Ioana Madalina Fenyo, Andra Todirita, Alina Constantin, Adrian Manea. High glucose-induced endothelin-1 expression is mediated by c-Src tyrosine kinase. Congresul National al Societatii Romane de Biologie Celulara cu Participare Internationala si A XXXII-A Sesiune Stiintifica Anuala, 2014. Bulletin of Romanian Society for Cell Biology, no. 42, pg. 98.
9. Adrian Manea, Simona-Adriana Manea, Ana-Maria Gan, Alina Constantin, Ioana Madalina Fenyo, Andra Todirita, Monica Raicu, Horia Muresian, Maya Simionescu. Human monocytes and macrophages express functional NADPH oxidase 5; a novel source of reactive oxygen species in atherosclerosis. Congresul National al Societatii Romane de Biologie Celulara cu Participare Internationala si A XXXII-A Sesiune Stiintifica Anuala, 2014. Bulletin of Romanian Society for Cell Biology, no. 42, pg. 96 (First prize for poster presentation).
10. Simona-Adriana Manea, Andra Todirita, Ioana Madalina Fenyo, Alina Constantin, Adrian Manea. c-Src tyrosine kinase mediates high glucose-induced endothelin-1 expression in diabetes. European Society of Cardiology Congress, 2014. European Heart Journal - Abstract Supplement, 2014, 35 (Abstract Supplement), P4441, pg. 788-789.
11. Adrian Manea, Andra Todirita, Alina Constantin, Simona-Adriana Manea. Epigenetic regulation of NADPH oxidase by histone acetylation in human aortic smooth muscle cells. European Society of Cardiology Congress, 2014. European Heart Journal - Abstract Supplement, 2014, 35 (Abstract Supplement), P3424, pg. 613.
12. Simona-Adriana Manea, Ioana Madalina Fenyo, Adrian Manea.Epigenetic regulation of endothelin-1 expression by histone acetylation/deacetylation in diabetes. 7th National Congress with International Participation and 33rd Annual Scientific Session of the RSCB, 2015. Bulletin of Romanian Society for Cell Biology,no. 43, pg. 71 (First prize for poster presentation).
13. Adrian Manea, Simona-Adriana Manea, Ioana Madalina Fenyo, Monica Raicu, Maya Simionescu. High glucose-induced NADPH oxidase expression and activity is mediated by epigenetic mechanisms in vascular smooth muscle cells. 7th National Congress with International Participation and 33rd Annual Scientific Session of the RSCB, 2015. Bulletin of Romanian Society for Cell Biology, no. 43, pg. 70.
14. Adrian Manea, Ioana Madalina Fenyo,Simona-Adriana Manea. High glucose-induced NADPH oxidase expression and activity is mediated by epigenetic mechanisms in vascular smooth muscle cells. 40th FEBS Congress, 2015. FEBS Journal 282(Suppl. 1) (2015) 55, pg. 65.

